Advancing stroke recovery: unlocking the potential of cellular dynamics in stroke recovery
- PMID: 38992073
- PMCID: PMC11239950
- DOI: 10.1038/s41420-024-02049-5
Advancing stroke recovery: unlocking the potential of cellular dynamics in stroke recovery
Abstract
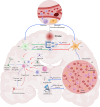
Stroke stands as a predominant cause of mortality and morbidity worldwide, and there is a pressing need for effective therapies to improve outcomes and enhance the quality of life for stroke survivors. In this line, effective efferocytosis, the clearance of apoptotic cells, plays a crucial role in neuroprotection and immunoregulation. This process involves specialized phagocytes known as "professional phagocytes" and consists of four steps: "Find-Me," "Eat-Me," engulfment/digestion, and anti-inflammatory responses. Impaired efferocytosis can lead to secondary necrosis and inflammation, resulting in adverse outcomes following brain pathologies. Enhancing efferocytosis presents a potential avenue for improving post-stroke recovery. Several therapeutic targets have been identified, including osteopontin, cysteinyl leukotriene 2 receptor, the µ opioid receptor antagonist β-funaltrexamine, and PPARγ and RXR agonists. Ferroptosis, defined as iron-dependent cell death, is now emerging as a novel target to attenuate post-stroke tissue damage and neuronal loss. Additionally, several biomarkers, most importantly CD163, may serve as potential biomarkers and therapeutic targets for acute ischemic stroke, aiding in stroke diagnosis and prognosis. Non-pharmacological approaches involve physical rehabilitation, hypoxia, and hypothermia. Mitochondrial dysfunction is now recognized as a major contributor to the poor outcomes of brain stroke, and medications targeting mitochondria may exhibit beneficial effects. These strategies aim to polarize efferocytes toward an anti-inflammatory phenotype, limit the ingestion of distressed but viable neurons, and stimulate efferocytosis in the late phase of stroke to enhance post-stroke recovery. These findings highlight promising directions for future research and development of effective stroke recovery therapies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Efferocytosis: A new therapeutic target for stroke.Chin Med J (Engl). 2024 Dec 5;137(23):2843-2850. doi: 10.1097/CM9.0000000000003363. Epub 2024 Nov 12. Chin Med J (Engl). 2024. PMID: 39528491 Free PMC article. Review.
-
Sigma-1 receptor-regulated efferocytosis by infiltrating circulating macrophages/microglial cells protects against neuronal impairments and promotes functional recovery in cerebral ischemic stroke.Theranostics. 2023 Jan 1;13(2):543-559. doi: 10.7150/thno.77088. eCollection 2023. Theranostics. 2023. PMID: 36632219 Free PMC article.
-
Efferocytosis in atherosclerotic lesions: Malfunctioning regulatory pathways and control mechanisms.Pharmacol Ther. 2018 Aug;188:12-25. doi: 10.1016/j.pharmthera.2018.02.003. Epub 2018 Feb 11. Pharmacol Ther. 2018. PMID: 29444453 Review.
-
Brain Cleanup as a Potential Target for Poststroke Recovery: The Role of RXR (Retinoic X Receptor) in Phagocytes.Stroke. 2020 Mar;51(3):958-966. doi: 10.1161/STROKEAHA.119.027315. Epub 2020 Jan 9. Stroke. 2020. PMID: 31914884 Free PMC article.
-
Efferocytosis and Atherosclerosis: Regulation of Phagocyte Function by MicroRNAs.Trends Endocrinol Metab. 2019 Sep;30(9):672-683. doi: 10.1016/j.tem.2019.07.006. Epub 2019 Aug 2. Trends Endocrinol Metab. 2019. PMID: 31383556 Review.
Cited by
-
Cinnamaldehyde and its combination with deferoxamine ameliorate inflammation, ferroptosis and hematoma expansion after intracerebral hemorrhage in mice.J Neuroinflammation. 2025 Feb 21;22(1):45. doi: 10.1186/s12974-025-03373-y. J Neuroinflammation. 2025. PMID: 39985048 Free PMC article.
-
Neuroglia and immune cells play different roles in neuroinflammation and neuroimmune response in post-stroke neural injury and repair.Acta Pharmacol Sin. 2025 Aug 12. doi: 10.1038/s41401-025-01640-5. Online ahead of print. Acta Pharmacol Sin. 2025. PMID: 40797113 Review.
-
Targeting PD-L1 for Ischemic Stroke Recovery: Age-Dependent Modulation of Immune and BBB Pathways.CNS Neurosci Ther. 2025 Jul;31(7):e70523. doi: 10.1111/cns.70523. CNS Neurosci Ther. 2025. PMID: 40702766 Free PMC article.
-
Genetic causality of lipidomic and immune cell profiles in ischemic stroke.Front Neurol. 2024 Sep 30;15:1437153. doi: 10.3389/fneur.2024.1437153. eCollection 2024. Front Neurol. 2024. PMID: 39403270 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

