Using torsional wave elastography to evaluate spring pot parameters in skin tumor mimicking phantoms
- PMID: 38992074
- PMCID: PMC11239839
- DOI: 10.1038/s41598-024-66621-w
Using torsional wave elastography to evaluate spring pot parameters in skin tumor mimicking phantoms
Erratum in
-
Author Correction: Using torsional wave elastography to evaluate spring pot parameters in skin tumor mimicking phantoms.Sci Rep. 2024 Aug 14;14(1):18864. doi: 10.1038/s41598-024-69708-6. Sci Rep. 2024. PMID: 39143149 Free PMC article. No abstract available.
-
Author Correction: Using torsional wave elastography to evaluate spring pot parameters in skin tumor mimicking phantoms.Sci Rep. 2024 Oct 24;14(1):25196. doi: 10.1038/s41598-024-76809-9. Sci Rep. 2024. PMID: 39448748 Free PMC article. No abstract available.
Abstract
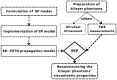
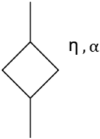
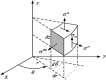
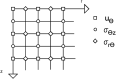
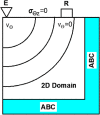
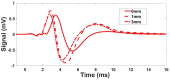
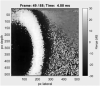
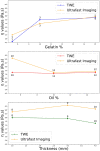
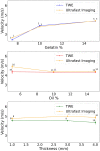
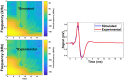
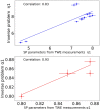
Estimating the tissue parameters of skin tumors is crucial for diagnosis and effective therapy in dermatology and related fields. However, identifying the most sensitive biomarkers require an optimal rheological model for simulating skin behavior this remains an ongoing research endeavor. Additionally, the multi-layered structure of the skin introduces further complexity to this task. In order to surmount these challenges, an inverse problem methodology, in conjunction with signal analysis techniques, is being employed. In this study, a fractional rheological model is presented to enhance the precision of skin tissue parameter estimation from the acquired signal from torsional wave elastography technique (TWE) on skin tumor-mimicking phantoms for lab validation and the estimation of the thickness of the cancerous layer. An exhaustive analysis of the spring-pot model (SP) solved by the finite difference time domain (FDTD) is conducted. The results of experiments performed using a TWE probe designed and prototyped in the laboratory were validated against ultrafast imaging carried out by the Verasonics Research System. Twelve tissue-mimicking phantoms, which precisely simulated the characteristics of skin tissue, were prepared for our experimental setting. The experimental data from these bi-layer phantoms were measured using a TWE probe, and the parameters of the skin tissue were estimated using inverse problem-solving. The agreement between the two datasets was evaluated by comparing the experimental data obtained from the TWE technique with simulated data from the SP- FDTD model using Pearson correlation, dynamic time warping (DTW), and time-frequency representation. Our findings show that the SP-FDTD model and TWE are capable of determining the mechanical properties of both layers in a bilayer phantom, using a single signal and an inverse problem approach. The ultrafast imaging and the validation of TWE results further demonstrate the robustness and reliability of our technology for a realistic range of phantoms. This fusion of the SP-FDTD model and TWE, as well as inverse problem-solving methods has the potential to have a considerable impact on diagnoses and treatments in dermatology and related fields.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- NORD (National Organization for Rare Disorders).
-
- Society, A. C. American Cancer Society.
-
- Stern, R. S. Prevalence of a History of Skin Cancer in 2007. Arch. Dermatol.146. 10.1001/archdermatol.2010.4 (2010). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

