Carotid artery transplantation of brain endothelial cells enhances neuroprotection and neurorepair in ischaemic stroke rats
- PMID: 38992118
- PMCID: PMC11579341
- DOI: 10.1038/s41401-024-01339-z
Carotid artery transplantation of brain endothelial cells enhances neuroprotection and neurorepair in ischaemic stroke rats
Abstract
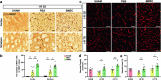
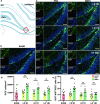
Brain microvascular endothelial cells (BMECs), an important component of the neurovascular unit, can promote angiogenesis and synaptic formation in ischaemic mice after brain parenchyma transplantation. Since the therapeutic efficacy of cell-based therapies depends on the extent of transplanted cell residence in the target tissue and cell migration ability, the delivery route has become a hot research topic. In this study, we investigated the effects of carotid artery transplantation of BMECs on neuronal injury, neurorepair, and neurological dysfunction in rats after cerebral ischaemic attack. Purified passage 1 endothelial cells (P1-BMECs) were prepared from mouse brain tissue. Adult rats were subjected to transient middle cerebral artery occlusion (MCAO) for 30 min. Then, the rats were treated with 5 × 105 P1-BMECs through carotid artery infusion or tail vein injection. We observed that carotid artery transplantation of BMECs produced more potent neuroprotective effects than caudal injection in MCAO rats, including reducing infarct size and alleviating neurological deficits in behavioural tests. Carotid artery-transplanted BMECs displayed a wider distribution in the ischaemic rat brain. Immunostaining for endothelial progenitor cells and the mature endothelial cell markers CD34 and RECA-1 showed that carotid artery transplantation of BMECs significantly increased angiogenesis. Carotid artery transplantation of BMECs significantly increased the number of surviving neurons, decreased the cerebral infarction volume, and alleviated neurological deficits. In addition, we found that carotid artery transplantation of BMECs significantly enhanced ischaemia-induced hippocampal neurogenesis, as measured by doublecortin (DCX) and Ki67 double staining within 2 weeks after ischaemic injury. We conclude that carotid artery transplantation of BMECs can promote cerebral angiogenesis, neurogenesis, and neurological function recovery in adult rats after ischaemic stroke. Our results suggest that carotid injection of BMECs may be a promising new approach for treating acute brain injuries.
Keywords: brain stroke; carotid artery transplantation; endothelial cell therapy; neurorepair; neurovascular unit.
© 2024. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. - PubMed
-
- Campbell BCV, Ma H, Ringleb PA, Parsons MW, Churilov L, Bendszus M, et al. Extending thrombolysis to 4.5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet. 2019; 394: 139–47. - PubMed
-
- Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 h after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

