Lipidome changes due to improved dietary fat quality inform cardiometabolic risk reduction and precision nutrition
- PMID: 38992128
- PMCID: PMC11485259
- DOI: 10.1038/s41591-024-03124-1
Lipidome changes due to improved dietary fat quality inform cardiometabolic risk reduction and precision nutrition
Abstract
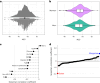
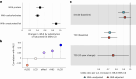
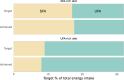
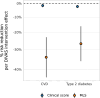
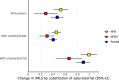
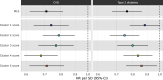
Current cardiometabolic disease prevention guidelines recommend increasing dietary unsaturated fat intake while reducing saturated fats. Here we use lipidomics data from a randomized controlled dietary intervention trial to construct a multilipid score (MLS), summarizing the effects of replacing saturated fat with unsaturated fat on 45 lipid metabolite concentrations. In the EPIC-Potsdam cohort, a difference in the MLS, reflecting better dietary fat quality, was associated with a significant reduction in the incidence of cardiovascular disease (-32%; 95% confidence interval (95% CI): -21% to -42%) and type 2 diabetes (-26%; 95% CI: -15% to -35%). We built a closely correlated simplified score, reduced MLS (rMLS), and observed that beneficial rMLS changes, suggesting improved dietary fat quality over 10 years, were associated with lower diabetes risk (odds ratio per standard deviation of 0.76; 95% CI: 0.59 to 0.98) in the Nurses' Health Study. Furthermore, in the PREDIMED trial, an olive oil-rich Mediterranean diet intervention primarily reduced diabetes incidence among participants with unfavorable preintervention rMLS levels, suggestive of disturbed lipid metabolism before intervention. Our findings indicate that the effects of dietary fat quality on the lipidome can contribute to a more precise understanding and possible prediction of the health outcomes of specific dietary fat modifications.
© 2024. The Author(s).
Conflict of interest statement
J.S.S. received grants through his institution and support for attending meetings/travel from the Nut and Dried Fruit Foundation. He is a nonpaid member of the Scientific Committee of the Nut and Dried fruit Foundation and the Scientific Committee of Danone Institute International and was a member of the Executive Committee of Danone Institute Spain. He received personal fees for serving as a Member of the Institute Danone Spain Advisory Board. J.A.L. was Deputy Chair of the UK Government’s Scientific Advisory Committee on Nutrition (SACN) and SACN’s Saturated Fat Working Group (2015–2019). C.W. has contributed to a precision nutrition seminar for the research and development department of Barilla G. e R. Fratelli S.p.A. (2023). The other authors declare no competing interests.
Figures





References
-
- Chatterjee, S., Khunti, K. & Davies, M. J. Type 2 diabetes. Lancet389, 2239–2251 (2017). - PubMed
-
- Zhou, X. et al. Cost-effectiveness of diabetes prevention interventions targeting high-risk individuals and whole populations: a systematic review. Diabetes Care43, 1593–1616 (2020). - PubMed
-
- Leong, D. P. et al. Reducing the global burden of cardiovascular disease, part 2: prevention and treatment of cardiovascular disease. Circ. Res.121, 695–710 (2017). - PubMed
-
- World Health Organization. Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children: WHO Guideline (World Health Organization, 2023). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

