Nuclear actin assembly is an integral part of decidualization in human endometrial stromal cells
- PMID: 38992143
- PMCID: PMC11239864
- DOI: 10.1038/s42003-024-06492-z
Nuclear actin assembly is an integral part of decidualization in human endometrial stromal cells
Abstract
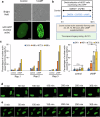
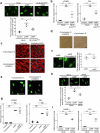
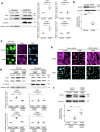
Decidualization of the human endometrium is critical for establishing pregnancy and is entailed by differentiation of endometrial stromal cells (ESCs) into decidual cells. During decidualization, the actin cytoskeleton is dynamically reorganized for the ESCs' morphological and functional changes. Although actin dynamically alters its polymerized state upon external stimuli not only in the cytoplasm, but also in the nucleus, nuclear actin dynamics during decidualization have not been elucidated. Here, we show that nuclear actin was specifically assembled during decidualization of human ESCs. This decidualization-specific formation of nuclear actin filaments was disassembled following the withdrawal of the decidualization stimulus, suggesting its reversible process. Mechanistically, RNA-seq analyses revealed that the forced disassembly of nuclear actin resulted in the suppression of decidualization, accompanied with the abnormal upregulation of cell proliferation genes, leading to incomplete cell cycle arrest. CCAAT/enhancer-binding protein beta (C/EBPβ), an important regulator for decidualization, was responsible for downregulation of the nuclear actin exporter, thus accelerating nuclear actin accumulation and its assembly for decidualization. Taken together, we demonstrate that decidualization-specific nuclear actin assembly induces cell cycle arrest for establishing the decidualized state of ESCs. We propose that not only the cytoplasmic actin, but also nuclear actin dynamics profoundly affect decidualization process in humans for ensuring pregnancy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
YTHDC1 Promoted Cell Proliferation and Decidualization by Maintaining Nuclear C/EBPβ Stability in Decidual Stromal Cells.Reprod Sci. 2025 Aug;32(8):2779-2792. doi: 10.1007/s43032-025-01888-6. Epub 2025 Jun 4. Reprod Sci. 2025. PMID: 40464838
-
Novel Function of a Transcription Factor WT1 in Regulating Decidualization in Human Endometrial Stromal Cells and Its Molecular Mechanism.Endocrinology. 2017 Oct 1;158(10):3696-3707. doi: 10.1210/en.2017-00478. Endocrinology. 2017. PMID: 28977591
-
Deciphering the role of PGRMC2 in the human endometrium during the menstrual cycle and in vitro decidualization using an in vitro approach.Hum Reprod. 2024 May 2;39(5):1042-1056. doi: 10.1093/humrep/deae044. Hum Reprod. 2024. PMID: 38452349
-
Endometrial stromal cells and decidualized stromal cells: origins, transformation and functions.Gene. 2014 Nov 1;551(1):1-14. doi: 10.1016/j.gene.2014.08.047. Epub 2014 Aug 26. Gene. 2014. PMID: 25168894 Review.
-
Glucose and lipid metabolisms in human endometrial stromal cells during decidualization.Endocr J. 2023 May 29;70(5):465-472. doi: 10.1507/endocrj.EJ23-0099. Epub 2023 Apr 18. Endocr J. 2023. PMID: 37081638 Review.
Cited by
-
Recent advances in nuclear actin research.Nucleus. 2025 Dec;16(1):2498643. doi: 10.1080/19491034.2025.2498643. Epub 2025 May 4. Nucleus. 2025. PMID: 40320716 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- JP21K09542/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP19H05751/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP20K21376/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP23H02399/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP20K18191/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

