Duo photoprotective effect via silica-coated zinc oxide nanoparticles and Vitamin C nanovesicles composites
- PMID: 38992234
- PMCID: PMC11263436
- DOI: 10.1007/s11095-024-03733-y
Duo photoprotective effect via silica-coated zinc oxide nanoparticles and Vitamin C nanovesicles composites
Abstract
Objective: Zinc Oxide nanoparticles (ZnO NPs) are used widely in nowadays personal care products, especially sunscreens, as a protector against UV irradiation. Yet, they have some reports of potential toxicity. Silica is widely used to cage ZnO NPs to reduce their potential toxicity. Vitamin C derivative, Magnesium Ascorpyl Phosphate (MAP), is a potent antioxidant that can efficiently protect human skin from harmful impacts of UV irradiation and oxidative stress. The combination of silica coated ZnO NPs and MAP nanovesicles could have potential synergistic protective effect against skin photodamage.
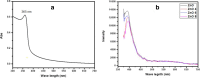
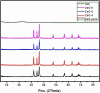
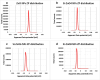
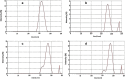
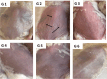
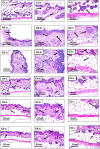
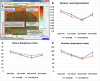
Methods: Silica coated ZnO NPs and MAP nanovesicles (ethosomes and niosomes) were synthesized, formulated, and evaluated as topical gels. These gel formulations were evaluated in mice for their photoprotective effect against UV irradiation through histopathology and immuno-histochemistry study. Split-face clinical study was conducted to compare the effect of application of silica coated ZnO NPs either alone or combined with MAP nanovesicles. Their photoprotective action was evaluated, using Antera 3D® camera, for melanin level, roughness index and wrinkles depth.
Results: Silica coated ZnO NPs when combined with MAP nanovesicles protected mice skin from UV irradiation and decreased the expression of the proinflammatory cytokines, NF-κB. Clinically, silica coated ZnO NPs, alone or combined with MAP nanovesicles, could have significant effect to decrease melanin level, roughness index and wrinkles depth with higher effect for the combination.
Conclusion: A composite of silica coated ZnO NPs and MAP nanovesicles could be a promising cosmetic formulation for skin protection against photodamage signs such as hyperpigmentation, roughness, and wrinkles.
Keywords: NF-κB; UV irradiation; Zno; antera 3D® camera; ethosomes; magnesium ascorbyl phosphate (MAP); niosomes; photodamage; silica.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Singh O, Singh MP, Kohli N, Singh RC. Effect of pH on the morphology and gas sensing properties of ZnO nanostructures. Sens Actuators B. 2012;166–167(402):438–43. 10.1016/j.snb.2012.02.085. 10.1016/j.snb.2012.02.085 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

