Western diet reduces small intestinal intraepithelial lymphocytes via FXR-Interferon pathway
- PMID: 38992433
- PMCID: PMC12317669
- DOI: 10.1016/j.mucimm.2024.07.001
Western diet reduces small intestinal intraepithelial lymphocytes via FXR-Interferon pathway
Abstract
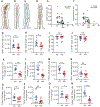
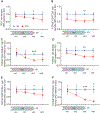
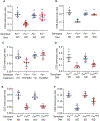
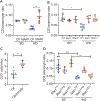
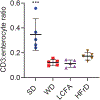
The prevalence of obesity in the United States has continued to increase over the past several decades. Understanding how diet-induced obesity modulates mucosal immunity is of clinical relevance. We previously showed that consumption of a high fat, high sugar "Western" diet (WD) reduces the density and function of small intestinal Paneth cells, a small intestinal epithelial cell type with innate immune function. We hypothesized that obesity could also result in repressed gut adaptive immunity. Using small intestinal intraepithelial lymphocytes (IEL) as a readout, we found that in non-inflammatory bowel disease (IBD) subjects, high body mass index correlated with reduced IEL density. We recapitulated this in wild type (WT) mice fed with WD. A 4-week WD consumption was able to reduce IEL but not splenic, blood, or bone marrow lymphocytes, and the effect was reversible after another 2 weeks of standard diet (SD) washout. Importantly, WD-associated IEL reduction was not dependent on the presence of gut microbiota, as WD-fed germ-free mice also showed IEL reduction. We further found that WD-mediated Farnesoid X Receptor (FXR) activation in the gut triggered IEL reduction, and this was partially mediated by intestinal phagocytes. Activated FXR signaling stimulated phagocytes to secrete type I IFN, and inhibition of either FXR or type I IFN signaling within the phagocytes prevented WD-mediated IEL loss. Therefore, WD consumption represses both innate and adaptive immunity in the gut. These findings have significant clinical implications in the understanding of how diet modulates mucosal immunity.
Keywords: Adaptive immunity; Fatty acid; Macrophages; Obesity.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: T. Stappenbeck advises Janssen, Boehringer Ingelheim, Kallyope, Takada, and Roche. T.C. Liu has research contracts with Denali Therapeutics and Interline Therapeutics. All other authors declare no relevant competing interests.
Figures


References
-
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief 2017; (288): 1–8. - PubMed
-
- Gruchala-Niedoszytko M, Malgorzewicz S, Niedoszytko M, Gnacinska M, Jassem E. The influence of obesity on inflammation and clinical symptoms in asthma. Adv Med Sci 2013; 58(1): 15–21. - PubMed
-
- Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 2017; 13(11): 633–643. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

