Regulatable assembly of synthetic microtubule architectures using engineered microtubule-associated protein-IDR condensates
- PMID: 38992434
- PMCID: PMC11342785
- DOI: 10.1016/j.jbc.2024.107544
Regulatable assembly of synthetic microtubule architectures using engineered microtubule-associated protein-IDR condensates
Abstract
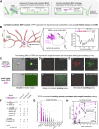
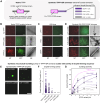
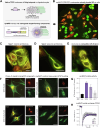
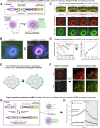
Microtubule filaments are assembled into higher-order structures using microtubule-associated proteins. However, synthetic MAPs that direct the formation of new structures are challenging to design, as nanoscale biochemical activities must be organized across micron length-scales. Here, we develop modular MAP-IDR condensates (synMAPs) that enable inducible assembly of higher-order microtubule structures for synthetic exploration in vitro and in mammalian cells. synMAPs harness a small microtubule-binding domain from oligodendrocytes (TPPP) whose activity we show can be rewired by interaction with unrelated condensate-forming IDR sequences. This combination is sufficient to allow synMAPs to self-organize multivalent structures that bind and bridge microtubules into higher-order architectures. By regulating the connection between the microtubule-binding domain and condensate-forming components of a synMAP, the formation of these structures can be triggered by small molecules or cell-signaling inputs. We systematically test a panel of synMAP circuit designs to define how the assembly of these synthetic microtubule structures can be controlled at the nanoscale (via microtubule-binding affinity) and microscale (via condensate formation). synMAPs thus provide a modular starting point for the design of higher-order microtubule systems and an experimental testbed for exploring condensate-directed mechanisms of higher-order microtubule assembly from the bottom-up.
Keywords: cell circuits; condensates; cytoskeleton; microtubules; synthetic biology.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


Update of
-
Regulatable assembly of synthetic microtubule architectures using engineered MAP-IDR condensates.bioRxiv [Preprint]. 2023 Dec 6:2023.03.14.532644. doi: 10.1101/2023.03.14.532644. bioRxiv. 2023. Update in: J Biol Chem. 2024 Aug;300(8):107544. doi: 10.1016/j.jbc.2024.107544. PMID: 38105997 Free PMC article. Updated. Preprint.
Similar articles
-
Regulatable assembly of synthetic microtubule architectures using engineered MAP-IDR condensates.bioRxiv [Preprint]. 2023 Dec 6:2023.03.14.532644. doi: 10.1101/2023.03.14.532644. bioRxiv. 2023. Update in: J Biol Chem. 2024 Aug;300(8):107544. doi: 10.1016/j.jbc.2024.107544. PMID: 38105997 Free PMC article. Updated. Preprint.
-
Overexpression of the microtubule-binding protein CLIP-170 induces a +TIP network superstructure consistent with a biomolecular condensate.PLoS One. 2021 Dec 10;16(12):e0260401. doi: 10.1371/journal.pone.0260401. eCollection 2021. PLoS One. 2021. PMID: 34890409 Free PMC article.
-
CLASP-mediated competitive binding in protein condensates directs microtubule growth.Nat Commun. 2024 Aug 2;15(1):6509. doi: 10.1038/s41467-024-50863-3. Nat Commun. 2024. PMID: 39095354 Free PMC article.
-
Recent advances in engineering synthetic biomolecular condensates.Biotechnol Adv. 2024 Dec;77:108452. doi: 10.1016/j.biotechadv.2024.108452. Epub 2024 Sep 11. Biotechnol Adv. 2024. PMID: 39271032 Review.
-
Learning physics and biology from cytoskeletal and condensate interactions.Curr Opin Cell Biol. 2025 Jun;94:102506. doi: 10.1016/j.ceb.2025.102506. Epub 2025 Apr 4. Curr Opin Cell Biol. 2025. PMID: 40184990 Review.
Cited by
-
Synthetic Forms Most Beautiful: Engineering Insights into Self-Organization.Physiology (Bethesda). 2025 Jul 1;40(4):0. doi: 10.1152/physiol.00064.2024. Epub 2025 Feb 12. Physiology (Bethesda). 2025. PMID: 39938118 Free PMC article. Review.
References
-
- Vale R.D. The molecular motor Toolbox for Intracellular Transport. Cell. 2003;112:467–480. - PubMed
-
- Nogales E. Structural insights into microtubule function. Annu. Rev. Biophys. Biomol. Struct. 2001;30:397–420. - PubMed
-
- Olmsted J.B. Microtubule-associated proteins. Annu. Rev. Cell Biol. 1986;2:421–457. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

