In Fanconi anemia, impaired accumulation of bone marrow neutrophils during emergency granulopoiesis induces hematopoietic stem cell stress
- PMID: 38992437
- PMCID: PMC11342097
- DOI: 10.1016/j.jbc.2024.107548
In Fanconi anemia, impaired accumulation of bone marrow neutrophils during emergency granulopoiesis induces hematopoietic stem cell stress
Abstract
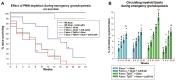
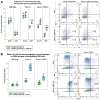
Fanconi anemia (FA) is an inherited disorder of DNA repair due to mutation in one of 20+ interrelated genes that repair intrastrand DNA crosslinks and rescue collapsed or stalled replication forks. The most common hematologic abnormality in FA is anemia, but progression to bone marrow failure (BMF), clonal hematopoiesis, or acute myeloid leukemia may also occur. In prior studies, we found that Fanconi DNA repair is required for successful emergency granulopoiesis; the process for rapid neutrophil production during the innate immune response. Specifically, Fancc-/- mice did not develop neutrophilia in response to emergency granulopoiesis stimuli, but instead exhibited apoptosis of bone marrow hematopoietic stem cells and differentiating neutrophils. Repeated emergency granulopoiesis challenges induced BMF in most Fancc-/- mice, with acute myeloid leukemia in survivors. In contrast, we found equivalent neutrophilia during emergency granulopoiesis in Fancc-/-Tp53+/- mice and WT mice, without BMF. Since termination of emergency granulopoiesis is triggered by accumulation of bone marrow neutrophils, we hypothesize neutrophilia protects Fancc-/-Tp53+/- bone marrow from the stress of a sustained inflammation that is experienced by Fancc-/- mice. In the current work, we found that blocking neutrophil accumulation during emergency granulopoiesis led to BMF in Fancc-/-Tp53+/- mice, consistent with this hypothesis. Blocking neutrophilia during emergency granulopoiesis in Fancc-/-Tp53+/- mice (but not WT) impaired cell cycle checkpoint activity, also found in Fancc-/- mice. Mechanisms for loss of cell cycle checkpoints during infectious disease challenges may define molecular markers of FA progression, or suggest therapeutic targets for bone marrow protection in this disorder.
Keywords: DNA damage response; DNA repair; cell cycle; hematopoiesis; inflammasome; innate immunity; neutrophil.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
TP53 Haploinsufficiency Rescues Emergency Granulopoiesis in FANCC-/- Mice.J Immunol. 2018 Mar 15;200(6):2129-2139. doi: 10.4049/jimmunol.1700931. Epub 2018 Feb 2. J Immunol. 2018. PMID: 29427417 Free PMC article.
-
Increased Fanconi C expression contributes to the emergency granulopoiesis response.J Clin Invest. 2013 Sep;123(9):3952-66. doi: 10.1172/JCI69032. Epub 2013 Aug 8. J Clin Invest. 2013. PMID: 23925293 Free PMC article.
-
Ruxolitinib ameliorates progressive anemia and improves survival during episodes of emergency granulopoiesis in Fanconi C-/- mice.Exp Hematol. 2022 May;109:55-67.e2. doi: 10.1016/j.exphem.2022.03.001. Epub 2022 Mar 9. Exp Hematol. 2022. PMID: 35278531 Free PMC article.
-
TNF-α signaling in Fanconi anemia.Blood Cells Mol Dis. 2014 Jan;52(1):2-11. doi: 10.1016/j.bcmd.2013.06.005. Epub 2013 Jul 24. Blood Cells Mol Dis. 2014. PMID: 23890415 Free PMC article. Review.
-
Why hematopoietic stem cells fail in Fanconi anemia: Mechanisms and models.Bioessays. 2025 Jan;47(1):e2400191. doi: 10.1002/bies.202400191. Epub 2024 Oct 25. Bioessays. 2025. PMID: 39460396 Review.
References
-
- Rosenberg P.S., Alter B.P., Ebell W. Cancer risks in fanconi anemia: findings from the German fanconi anemia Registry. Haematologica. 2008;93:511–517. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

