Isolation methods and characterization of primary rat neurovascular cells
- PMID: 38992711
- PMCID: PMC11241874
- DOI: 10.1186/s13036-024-00434-3
Isolation methods and characterization of primary rat neurovascular cells
Abstract
Background: There is significant interest in isolating cells of the blood-brain barrier (BBB) for use in in vitro screening of therapeutics and analyzing cell specific roles in neurovascular pathology. Primary brain cells play an advantageous role in BBB models; however, isolation procedures often do not produce cells at high enough yields for experiments. In addition, although numerous reports provide primary cell isolation methods, the field is lacking in documentation and detail of expected morphological changes that occur throughout culturing and there are minimal troubleshooting resources. Here, we present simplified, robust, and reproducible methodology for isolating astrocytes, pericytes, and endothelial cells, and demonstrate several morphological benchmarks for each cell type throughout the process and culture timeframe. We also analyze common considerations for developing neurovascular cell isolation procedures and recommend solutions for troubleshooting.
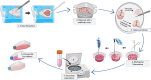
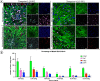
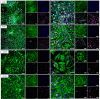
Results: The presented methodology isolated astrocytes, pericytes, and endothelial cells and enabled cell attachment, maturation, and cell viability. We characterized milestones in cell maturation over 12 days in culture, a common timeline for applications of these cell types in BBB models. Phase contrast microscopy was used to show initial cell plating, attachment, and daily growth of isolated cells. Confocal microscopy images were analyzed to determine the identity of cell types and changes to cell morphology. Nuclear staining was also used to show the viability and proliferation of glial cells at four time points. Astrocyte branches became numerous and complex with increased culture time. Microglia, oligodendrocytes, and neurons were present in mixed glial cultures for 12 days, though the percentage of microglia and neurons expectedly decreased after passaging, with microglia demonstrating a less branched morphology.
Conclusions: Neurovascular cells can be isolated through our optimized protocols that minimize cell loss and encourage the adhesion and proliferation of isolated cells. By identifying timepoints of viable glia and neurons within an astrocyte-dominant mixed culture, these cells can be used to evaluate drug targeting, uptake studies, and response to pathological stimulus in the neurovascular unit.
Keywords: Astrocytes; Endothelial cells; Pericytes; Primary blood-brain barrier cell isolation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes.Neurochem Int. 2009 Mar-Apr;54(3-4):253-63. doi: 10.1016/j.neuint.2008.12.002. Epub 2008 Dec 7. Neurochem Int. 2009. PMID: 19111869
-
A novel brain neurovascular unit model with neurons, astrocytes and microvascular endothelial cells of rat.Int J Biol Sci. 2013;9(2):174-89. doi: 10.7150/ijbs.5115. Epub 2013 Feb 7. Int J Biol Sci. 2013. PMID: 23412420 Free PMC article.
-
A flow cytometry-based protocol for syngenic isolation of neurovascular unit cells from mouse and human tissues.Nat Protoc. 2023 May;18(5):1510-1542. doi: 10.1038/s41596-023-00805-y. Epub 2023 Mar 1. Nat Protoc. 2023. PMID: 36859615 Review.
-
A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier.Lab Chip. 2017 Jan 31;17(3):448-459. doi: 10.1039/c6lc00638h. Lab Chip. 2017. PMID: 28001148
-
Targetability of the neurovascular unit in inflammatory diseases of the central nervous system.Immunol Rev. 2022 Oct;311(1):39-49. doi: 10.1111/imr.13121. Epub 2022 Jul 31. Immunol Rev. 2022. PMID: 35909222 Free PMC article. Review.
References
-
- Takata F, Dohgu S, Yamauchi A, Matsumoto J, Machida T, Fujishita K, et al. In Vitro Blood-Brain Barrier models using Brain Capillary endothelial cells isolated from neonatal and adult rats retain Age-Related Barrier properties. PLoS ONE. 2013;8(1):e55166. doi: 10.1371/journal.pone.0055166. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

