Modulating Weak Protein-Protein Cross-Interactions by the Addition of Free Amino Acids at Millimolar Concentrations
- PMID: 38992922
- PMCID: PMC11284779
- DOI: 10.1021/acs.jpcb.4c01086
Modulating Weak Protein-Protein Cross-Interactions by the Addition of Free Amino Acids at Millimolar Concentrations
Abstract
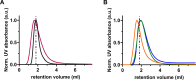
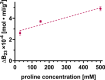
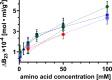
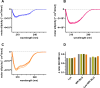
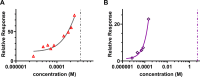
In this paper, we quantify weak protein-protein interactions in solution using cross-interaction chromatography (CIC) and surface plasmon resonance (SPR) and demonstrate that they can be modulated by the addition of millimolar concentrations of free amino acids. With CIC, we determined the second osmotic virial cross-interaction coefficient (B23) as a proxy for the interaction strength between two different proteins. We perform SPR experiments to establish the binding affinity between the same proteins. With CIC, we show that the amino acids proline, glutamine, and arginine render the protein cross-interactions more repulsive or equivalently less attractive. Specifically, we measured B23 between lysozyme (Lys) and bovine serum albumin (BSA) and between Lys and protein isolates (whey and canola). We find that B23 increases when amino acids are added to the solution even at millimolar concentrations, corresponding to protein/ligand stoichiometric ratios as low as 1:1. With SPR, we show that the binding affinity between proteins can change by 1 order of magnitude when 10 mM glutamine is added. In the case of Lys and one whey protein isolate (WPI), it changes from the mM to the M range, thus by 3 orders of magnitude. Interestingly, this efficient modulation of the protein cross-interactions does not alter the protein's secondary structure. The capacity of amino acids to modulate protein cross-interactions at mM concentrations is remarkable and may have an impact across fields in particular for specific applications in the food or pharmaceutical industries.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Electrophoresis.2025 Jul 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36251838 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Orthodontic treatment for crowded teeth in children.Cochrane Database Syst Rev. 2021 Dec 31;12(12):CD003453. doi: 10.1002/14651858.CD003453.pub2. Cochrane Database Syst Rev. 2021. PMID: 34970995 Free PMC article.
Cited by
-
Amino acids modulate liquid-liquid phase separation in vitro and in vivo by regulating protein-protein interactions.Proc Natl Acad Sci U S A. 2024 Dec 10;121(50):e2407633121. doi: 10.1073/pnas.2407633121. Epub 2024 Dec 6. Proc Natl Acad Sci U S A. 2024. PMID: 39642205 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

