Honey and levodopa comparably preserved substantia nigra pars compacta neurons through the modulation of nuclear factor erythroid 2-related factor 2 signaling pathway in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease model
- PMID: 38992924
- PMCID: PMC11424567
- DOI: 10.5115/acb.24.034
Honey and levodopa comparably preserved substantia nigra pars compacta neurons through the modulation of nuclear factor erythroid 2-related factor 2 signaling pathway in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease model
Abstract
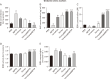
Parkinson's disease (PD) affects about 8.5 million individuals worldwide. Oxidative and inflammatory cascades are implicated in the neurological sequels, that are mostly unresolved in PD treatments. However, proper nutrition offers one of the most effective and least costly ways to decrease the burden of many diseases and their associated risk factors. Moreover, prevention may be the best response to the progressive nature of PD, thus, the therapeutic novelty of honey and levodopa may be prospective. This study aimed to investigate the neuroprotective role of honey and levodopa against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced oxidative stress. Fifty-four adult male Swiss mice were divided into control and PD model groups of 27 mice. Each third of the control mice either received phosphate buffered saline, honey, or levodopa for 21 days. However, each third of the PD models was either pretreated with honey and levodopa or not pretreated. Behavioral studies and euthanasia were conducted 2 and 8 days after MPTP administration respectively. The result showed that there were significantly (P<0.05) higher motor activities in the PD models pretreated with the honey as well as levodopa. furthermore, the pretreatments protected the midbrain against the chromatolysis and astrogliosis induced by MPTP. The expression of antioxidant markers (glutathione [GSH] and nuclear factor erythroid 2-related factor 2 [Nrf2]) was also significantly upregulated in the pretreated PD models. It is thus concluded that honey and levodopa comparably protected the substantia nigra pars compacta neurons against oxidative stress by modulating the Nrf2 signaling molecule thereby increasing GSH level to prevent MPTP-induced oxidative stress.
Keywords: 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine; Honey; Levodopa; Nrf2; Parkinson disease.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures




Similar articles
-
Vitamin E Analog Trolox Attenuates MPTP-Induced Parkinson's Disease in Mice, Mitigating Oxidative Stress, Neuroinflammation, and Motor Impairment.Int J Mol Sci. 2023 Jun 9;24(12):9942. doi: 10.3390/ijms24129942. Int J Mol Sci. 2023. PMID: 37373089 Free PMC article.
-
Early signs of neuronal apoptosis in the substantia nigra pars compacta of the progressive neurodegenerative mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid model of Parkinson's disease.Neuroscience. 2006 Jun 19;140(1):67-76. doi: 10.1016/j.neuroscience.2006.02.007. Epub 2006 Mar 14. Neuroscience. 2006. PMID: 16533572
-
Role of estrogen and levodopa in 1-methyl-4-pheny-l-1, 2, 3, 6-tetrahydropyridine (mptp)-induced cognitive deficit in Parkinsonian ovariectomized mice model: A comparative study.J Chem Neuroanat. 2017 Nov;85:50-59. doi: 10.1016/j.jchemneu.2017.07.002. Epub 2017 Jul 12. J Chem Neuroanat. 2017. PMID: 28711564
-
The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson's disease.Gene. 2013 Dec 10;532(1):18-23. doi: 10.1016/j.gene.2013.07.085. Epub 2013 Aug 15. Gene. 2013. PMID: 23954870 Review.
-
The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson's disease.Curr Pharm Des. 2011;17(5):489-507. doi: 10.2174/138161211795164095. Curr Pharm Des. 2011. PMID: 21375482 Review.
References
-
- World Health Organization (WHO), author Ageing and health [Internet] WHO; 2022. Oct 1, [cited 2023 Dec 29]. Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health .
LinkOut - more resources
Full Text Sources
Miscellaneous
