Glucose prevents the acquisition of the capacitated state in pig spermatozoa
- PMID: 38993010
- PMCID: PMC11867919
- DOI: 10.1111/andr.13691
Glucose prevents the acquisition of the capacitated state in pig spermatozoa
Abstract
Background: Mammalian spermatozoa need to undergo a process named capacitation to be able to fertilize an oocyte. During their journey in the female tract, spermatozoa obtain energy while exposed to a changing environment containing a variety of metabolic substrates. The energy requirements for sperm capacitation are species-specific. In addition, the available energy source can hinder the process of sperm capacitation and eventually the acrosome reaction.
Objectives: To evaluate whether the metabolic substrates available in the in vitro sperm capacitation medium allow or interfere with the pig sperm capacitation process.
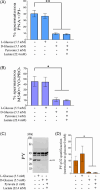
Material and methods: The effect of different metabolic substrates on sperm capacitation process was evaluated by analyzing phosphorylation in the p32 protein; the acrosome reaction and the ATP intracellular content.
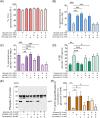
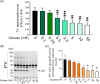
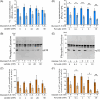
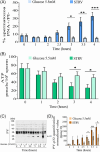
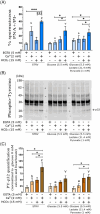
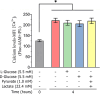
Results: The presence of glucose in the in vitro capacitating medium diminishes, in a concentration-dependent manner, parameters associated with the capacitated status: induced acrosome exocytosis, plasma membrane destabilization, and protein tyrosine phosphorylation. Conversely, sperm incubation with pyruvate or lactate, either individually or in combination, allows the attainment of the capacitated status. Unexpectedly, pig spermatozoa incubated without any extracellular energy substrates or with a non-metabolizable substrate (l-glucose) for 4 h displayed similar sperm viability to the control and exhibited a capacitated phenotype. The capacitation-like phenotype observed in starved pig spermatozoa (absence of glucose, lactate, and pyruvate) was dependent on extracellular bicarbonate and calcium levels, and these spermatozoa exhibited lower intracellular ATP content compared to those not capacitated. Nevertheless, the intracellular content of calcium was not modified in comparison to the control.
Discussion and conclusions: Our findings suggest that the metabolic substrates used to fuel pig sperm metabolism are important in achieving the capacitated status. The results of this work could be used to refine the capacitating medium employed in pig in vitro fertilization.
Keywords: acrosome reaction; capacitation; glucose; metabolic substrate; spermatozoa; starvation; tyrosine phosphorylation.
© 2024 The Author(s). Andrology published by Wiley Periodicals LLC on behalf of American Society of Andrology and European Academy of Andrology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Visconti PE. Sperm bioenergetics in a nutshell. Biol Reprod. 2012;87(3):72. https://doi.org10.1095/biolreprod.112.104109 - PMC - PubMed
-
- Hugentobler SA, Humpherson PG, Leese HJ, Sreenan JM, Morris DG. Energy substrates in bovine oviduct and uterine fluid and blood plasma during the oestrous cycle. Mol Reprod Dev. 2008;75(3):496‐503. https://doi.org10.1002/mrd.20760 - PubMed
-
- Harris SE, Gopichandran N, Picton HM, Leese HJ, Orsi NM. Nutrient concentrations in murine follicular fluid and the female reproductive tract. Theriogenology. 2005;64(4):992‐1006. https://doi.org10.1016/j.theriogenology.2005.01.004 - PubMed
-
- Nichol R, Hunter RH, Gardner DK, Leese HJ, Cooke GM. Concentrations of energy substrates in oviductal fluid and blood plasma of pigs during the peri‐ovulatory period. J Reprod Fertil. 1992;96(2):699‐707. https://doi.org10.1530/jrf.0.0960699 - PubMed
-
- González‐Fernández L, Sánchez‐Calabuig MJ, Calle‐Guisado V, et al. Stage‐specific metabolomic changes in equine oviductal fluid: new insights into the equine fertilization environment. Theriogenology. 2020;143:35‐43. https://doi.org10.1016/j.theriogenology.2019.11.035 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

