Spermatogonial stem cell technologies: applications from human medicine to wildlife conservation†
- PMID: 38993049
- PMCID: PMC11473898
- DOI: 10.1093/biolre/ioae109
Spermatogonial stem cell technologies: applications from human medicine to wildlife conservation†
Abstract
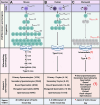
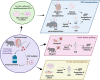
Spermatogonial stem cell (SSC) technologies that are currently under clinical development to reverse human infertility hold the potential to be adapted and applied for the conservation of endangered and vulnerable wildlife species. The biobanking of testis tissue containing SSCs from wildlife species, aligned with that occurring in pediatric human patients, could facilitate strategies to improve the genetic diversity and fitness of endangered populations. Approaches to utilize these SSCs could include spermatogonial transplantation or testis tissue grafting into a donor animal of the same or a closely related species, or in vitro spermatogenesis paired with assisted reproduction approaches. The primary roadblock to progress in this field is a lack of fundamental knowledge of SSC biology in non-model species. Herein, we review the current understanding of molecular mechanisms controlling SSC function in laboratory rodents and humans, and given our particular interest in the conservation of Australian marsupials, use a subset of these species as a case-study to demonstrate gaps-in-knowledge that are common to wildlife. Additionally, we review progress in the development and application of SSC technologies in fertility clinics and consider the translation potential of these techniques for species conservation pipelines.
Keywords: in vitro spermatogenesis; male infertility; marsupials; spermatogonial stem cells; spermatogonial transplantation; stem cell technologies; testis grafting; wildlife conservation.
© The Author(s) 2024. Published by Oxford University Press behalf of Society for the Study of Reproduction.
Figures



References
-
- Dobrinski I, Travis AJ. Germ cell transplantation for the propagation of companion animals, non-domestic and endangered species. Reprod Fertil Dev 2007; 19:732–739. - PubMed
-
- Johnston SD, Satake N, Zee Y, López-Fernández C, Holt WV, Gosálvez J. Osmotic stress and cryoinjury of koala sperm: an integrative study of the plasma membrane, chromatin stability and mitochondrial function. Reproduction 2012; 143:787–797. - PubMed
-
- Lam SS, Waugh C, Peng W, Sonne C. Wildfire puts koalas at risk of extinction. Science 2020; 367United States:750. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

