Pharmacological targeting cGAS/STING/NF-κB axis by tryptanthrin induces microglia polarization toward M2 phenotype and promotes functional recovery in a mouse model of spinal cord injury
- PMID: 38993129
- PMCID: PMC11881704
- DOI: 10.4103/NRR.NRR-D-23-01256
Pharmacological targeting cGAS/STING/NF-κB axis by tryptanthrin induces microglia polarization toward M2 phenotype and promotes functional recovery in a mouse model of spinal cord injury
Abstract
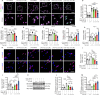
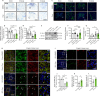
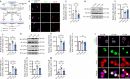
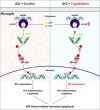
JOURNAL/nrgr/04.03/01300535-202511000-00031/figure1/v/2024-12-20T164640Z/r/image-tiff The M1/M2 phenotypic shift of microglia after spinal cord injury plays an important role in the regulation of neuroinflammation during the secondary injury phase of spinal cord injury. Regulation of shifting microglia polarization from M1 (neurotoxic and proinflammatory type) to M2 (neuroprotective and anti-inflammatory type) after spinal cord injury appears to be crucial. Tryptanthrin possesses an anti-inflammatory biological function. However, its roles and the underlying molecular mechanisms in spinal cord injury remain unknown. In this study, we found that tryptanthrin inhibited microglia-derived inflammation by promoting polarization to the M2 phenotype in vitro . Tryptanthrin promoted M2 polarization through inactivating the cGAS/STING/NF-κB pathway. Additionally, we found that targeting the cGAS/STING/NF-κB pathway with tryptanthrin shifted microglia from the M1 to M2 phenotype after spinal cord injury, inhibited neuronal loss, and promoted tissue repair and functional recovery in a mouse model of spinal cord injury. Finally, using a conditional co-culture system, we found that microglia treated with tryptanthrin suppressed endoplasmic reticulum stress-related neuronal apoptosis. Taken together, these results suggest that by targeting the cGAS/STING/NF-κB axis, tryptanthrin attenuates microglia-derived neuroinflammation and promotes functional recovery after spinal cord injury through shifting microglia polarization to the M2 phenotype.
Copyright © 2025 Neural Regeneration Research.
Conflict of interest statement
Figures



References
-
- Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. - PubMed
-
- Ban M, Su H, Zeng X, Chen C, Zhou S, Chen X, Nong Z. An active fraction from Spatholobus suberectus dunn inhibits the inflammatory response by regulating microglia activation, switching microglia polarization from M1 to M2 and suppressing the TLR4/MyD88/NF-κB pathway in LPS-stimulated BV2 cells. Heliyon. 2023;9:e14979. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

