Mesenchymal stromal cells protect combined oncolytic and helper-dependent adenoviruses from humoral immunity
- PMID: 38993326
- PMCID: PMC11238183
- DOI: 10.1016/j.omtm.2024.101279
Mesenchymal stromal cells protect combined oncolytic and helper-dependent adenoviruses from humoral immunity
Abstract
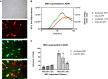
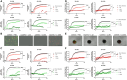
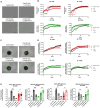
Systemic delivery of oncolytic and immunomodulatory adenoviruses may be required for optimal effects on human malignancies. Mesenchymal stromal cells (MSCs) can serve as delivery systems for cancer therapeutics due to their ability to transport and shield these agents while homing to tumors. We now use MSCs to deliver a clinically validated binary oncolytic and helper-dependent adenovirus combination (CAdVEC) to tumor cells. We show successful oncolysis and helper-dependent virus function in tumor cells even in the presence of plasma from adenovirus-seropositive donors. In both two- and three-dimensional cultures, CAdVEC function is eliminated even at high dilutions of seropositive plasma but is well sustained when CAdVEC is delivered by MSCs. These results provide a robust in vitro model to measure oncolytic and helper-dependent virus spread and demonstrate a beneficial role of using MSCs for systemic delivery of CAdVEC even in the presence of a neutralizing humoral response.
Keywords: helper-dependent adenovirus; mesenchymal stromal cells; oncolytic adenovirus; solid tumors; three-dimensional tumor spheroids.
© 2024 The Author(s).
Conflict of interest statement
M.K.B. serves on advisory boards for Marker Therapeutics, Allogene, Walking Fish, Abintus, Athenex-Kuur, Onk Therapeutics, Coya Therapeutics, Triumvira, Adaptimmune, Vor Therapeutics, and Tscan. M.K.B has equity in Allovir, Tessa Therapeutic Ltd, and Marker Therapeutics. M.K.B. has royalties from Takeda and Bellicum. M.S. received research funding from Tessa Therapeutic Ltd. and AstraZeneca. M.S. was a scientific consultant for Tessa Therapeutic Ltd.
Figures
References
-
- Wang D., Porter C.E., Lim B., Rosewell Shaw A., Robertson C.S., Woods M.L., Xu Y., Biegert G.G.W., Morita D., Wang T., et al. Ultralow-dose binary oncolytic/helper-dependent adenovirus promotes antitumor activity in preclinical and clinical studies. Sci. Adv. 2023;9 doi: 10.1126/sciadv.ade6790. - DOI - PMC - PubMed
-
- Rosewell Shaw A., Porter C.E., Watanabe N., Tanoue K., Sikora A., Gottschalk S., Brenner M.K., Suzuki M. Adenovirotherapy Delivering Cytokine and Checkpoint Inhibitor Augments CAR T Cells against Metastatic Head and Neck Cancer. Mol. Ther. 2017;25:2440–2451. doi: 10.1016/j.ymthe.2017.09.010. - DOI - PMC - PubMed
-
- Havunen R., Santos J.M., Sorsa S., Rantapero T., Lumen D., Siurala M., Airaksinen A.J., Cervera-Carrascon V., Tähtinen S., Kanerva A., Hemminki A. Abscopal Effect in Non-injected Tumors Achieved with Cytokine-Armed Oncolytic Adenovirus. Mol. Ther. Oncolytics. 2018;11:109–121. doi: 10.1016/j.omto.2018.10.005. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

