Metronidazole-Induced Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome With Parvovirus B19 Reactivation: A Pediatric Case
- PMID: 38993456
- PMCID: PMC11238016
- DOI: 10.7759/cureus.62125
Metronidazole-Induced Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome With Parvovirus B19 Reactivation: A Pediatric Case
Abstract
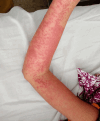
Drug reaction with eosinophilia and systemic symptoms (DRESS) is a severe and rare syndrome that causes life-threatening organ dysfunctions. Here, we present the case of a 10-year-old child who developed a pruritic erythematous eruption, fever, facial edema, and lymphadenopathy seven days after receiving intravenous metronidazole (20 mg/kg/day), vancomycin (50 mg/kg/day), and cefotaxime (200 mg/kg/day). Laboratory tests showed eosinophilia and liver damage as well as positive parvovirus B19 IgM and IgG indicating viral reactivation. Vancomycin was initially discontinued and later reintroduced with no ill effects. The patient was managed with topical corticosteroid emollients and cetirizine and improved within seven days of metronidazole withdrawal. Treatment with cefotaxime was continued and showed no adverse effects.
Keywords: dress and viruses; dress syndrome; drug reaction with eosinophilia and systemic symptoms; metronidazole side effects; parvovirus b-19; pediatrics; pharmaco-vigilance; pharmacology.
Copyright © 2024, Dridi et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- The variable clinical picture of drug-induced hypersensitivity syndrome/drug rash with eosinophilia and systemic symptoms in relation to the eliciting drug. Kano Y, Shiohara T. Immunol Allergy Clin North Am. 2009;29:481–501. - PubMed
-
- Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. Choudhary S, McLeod M, Torchia D, Romanelli P. http://pubmed.ncbi.nlm.nih.gov/23882307/ J Clin Aesthet Dermatol. 2013;6:31–37. - PMC - PubMed
-
- Drug reaction with eosinophilia and systemic symptoms (DRESS) in the pediatric population: a systematic review of the literature. Kim GY, Anderson KR, Davis DM, Hand JL, Tollefson MM. J Am Acad Dermatol. 2020;83:1323–1330. - PubMed
-
- Severe cutaneous adverse drug reactions in pediatric patients: a multicenter study. Dibek Misirlioglu E, Guvenir H, Bahceci S, et al. J Allergy Clin Immunol Pract. 2017;5:757–763. - PubMed
-
- A method for estimating the probability of adverse drug reactions. Naranjo CA, Busto U, Sellers EM, et al. Clin Pharmacol Ther. 1981;30:239–245. - PubMed
Publication types
LinkOut - more resources
Full Text Sources