No colonization resistance to Campylobacter jejuni in broilers fed brown algal extract-supplemented diets
- PMID: 38993493
- PMCID: PMC11236747
- DOI: 10.3389/fmicb.2024.1396949
No colonization resistance to Campylobacter jejuni in broilers fed brown algal extract-supplemented diets
Abstract
Introduction: Campylobacter jejuni gastroenteritis is the most commonly reported zoonosis within the EU, with poultry products regarded as the primary source of transmission to humans. Therefore, finding strategies to reduce Campylobacter colonization in broilers holds importance for public health. Recent studies suggest that supplementation of broiler feed with brown algal extracts, particularly laminarin, can provide beneficial effects on broiler gut health, growth performance, and gut microbiota. However, its effect on gut microbiota development and subsequent reduction of Campylobacter loads in broiler caeca during the later stages of the birds' lives remains unclear.
Methods: Experimental colonization of Ross 308 broilers with two different strains of C. jejuni was conducted, with groups fed either a basal diet or the same basal diet supplemented with 725 ppm algal extract from Saccharina latissima to provide 290 ppm laminarin. Fecal samples were collected for bacterial enumeration, and caecal samples were obtained before and after the C. jejuni challenge for the determination of microbiota development.
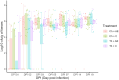
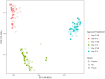
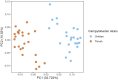
Results and discussion: No significant differences in fecal C. jejuni concentrations between the groups fed different diets or exposed to different C. jejuni strains were observed. This suggests that both strains colonized the birds equally well and that the laminarin rich algal extract did not have any inhibitory effect on C. jejuni colonization. Notably, 16S rRNA amplicon sequencing revealed detailed data on the caecal microbiota development, likely influenced by both bird age and C. jejuni colonization, which can be valuable for further development of broiler feed formulations aimed at promoting gut health.
Keywords: Campylobacter jejuni; Saccharina latissima; broiler; brown algae; host specificity; laminarin; microbiota.
Copyright © 2024 Eliasson, Sun, Cervin, Pavia, Tällberg, Ellström and Ivarsson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Intestinal colonization with Campylobacter jejuni affects broiler gut microbiota composition but is not inhibited by daily intake of Lactiplantibacillus plantarum.Front Microbiol. 2023 Jul 28;14:1205797. doi: 10.3389/fmicb.2023.1205797. eCollection 2023. Front Microbiol. 2023. PMID: 37577431 Free PMC article.
-
Fecal Microbiota Transplantation Reduces Campylobacter jejuni Colonization in Young Broiler Chickens Challenged by Oral Gavage but Not by Seeder Birds.Antibiotics (Basel). 2023 Oct 2;12(10):1503. doi: 10.3390/antibiotics12101503. Antibiotics (Basel). 2023. PMID: 37887204 Free PMC article.
-
Medium chain fatty acid feed supplementation reduces the probability of Campylobacter jejuni colonization in broilers.Vet Microbiol. 2010 Jul 14;143(2-4):314-8. doi: 10.1016/j.vetmic.2009.11.029. Epub 2009 Nov 27. Vet Microbiol. 2010. PMID: 20022713 Clinical Trial.
-
Colonization factors of Campylobacter jejuni in the chicken gut.Vet Res. 2011 Jun 29;42(1):82. doi: 10.1186/1297-9716-42-82. Vet Res. 2011. PMID: 21714866 Free PMC article. Review.
-
Campylobacter jejuni in Poultry: Pathogenesis and Control Strategies.Microorganisms. 2022 Oct 28;10(11):2134. doi: 10.3390/microorganisms10112134. Microorganisms. 2022. PMID: 36363726 Free PMC article. Review.
References
-
- Anderson M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austr. Ecol. 26, 32–46. 10.1111/j.1442-9993.2001.01070.pp.x - DOI
-
- Aviagen . (2019). Ross Nutrition Specifications. Available olnline aa: https://www.cipa.com.co/wp-content/uploads/2019/11/RossBroilerNutritionS... (accessed November 24, 2023).
-
- Awad W. A., Mann E., Dzieciol M., Hess C., Schmitz-Esser S., Wagner M., et al. (2016). Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with Campylobacter jejuni infection. Front. Cell. Infect. Microbiol. 6:154. 10.3389/fcimb.2016.00154 - DOI - PMC - PubMed
-
- Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48. 10.18637/jss.v067.i01 - DOI
LinkOut - more resources
Full Text Sources

