Role of inflammation and evidence for the use of colchicine in patients with acute coronary syndrome
- PMID: 38993522
- PMCID: PMC11236697
- DOI: 10.3389/fcvm.2024.1356023
Role of inflammation and evidence for the use of colchicine in patients with acute coronary syndrome
Abstract
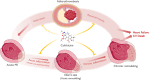
Acute Coronary Syndrome (ACS) significantly contributes to cardiovascular death worldwide. ACS may arise from the disruption of an atherosclerotic plaque, ultimately leading to acute ischemia and myocardial infarction. In the pathogenesis of atherosclerosis, inflammation assumes a pivotal role, not solely in the initiation and complications of atherosclerotic plaque formation, but also in the myocardial response to ischemic insult. Acute inflammatory processes, coupled with time to reperfusion, orchestrate ischemic and reperfusion injuries, dictating infarct magnitude and acute left ventricular (LV) remodeling. Conversely, chronic inflammation, alongside neurohumoral activation, governs persistent LV remodeling. The interplay between chronic LV remodeling and recurrent ischemic episodes delineates the progression of the disease toward heart failure and cardiovascular death. Colchicine exerts anti-inflammatory properties affecting both the myocardium and atherosclerotic plaque by modulating the activity of monocyte/macrophages, neutrophils, and platelets. This modulation can potentially result in a more favorable LV remodeling and forestalls the recurrence of ACS. This narrative review aims to delineate the role of inflammation across the different phases of ACS pathophysiology and describe the mechanistic underpinnings of colchicine, exploring its purported role in modulating each of these stages.
Keywords: NETosis; acute coronary syndrome; colchicine; inflammasome; myocardial infarction.
© 2024 Bulnes, González, Velásquez, Orellana, Venturelli and Martínez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Collaborators GBDCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392(10159):1736–88. 10.1016/S0140-6736(18)32203-7 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

