Cigarette smoke promotes IL-6-dependent lung cancer migration and osteolytic bone metastasis
- PMID: 38993553
- PMCID: PMC11234207
- DOI: 10.7150/ijbs.94339
Cigarette smoke promotes IL-6-dependent lung cancer migration and osteolytic bone metastasis
Abstract
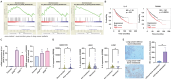
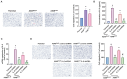
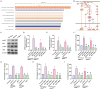
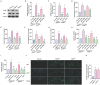
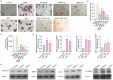
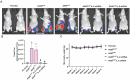
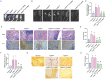
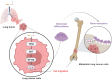
Lung cancer stands as a major contributor to cancer-related fatalities globally, with cigarette smoke playing a pivotal role in its development and metastasis. Cigarette smoke is also recognized as a risk factor for bone loss disorders like osteoporosis. However, the association between cigarette smoke and another bone loss disorder, lung cancer osteolytic bone metastasis, remains largely uncertain. Our Gene Set Enrichment Analysis (GSEA) indicated that smokers among lung cancer patients exhibited higher expression levels of bone turnover gene sets. Both The Cancer Genome Atlas (TCGA) database and our clinic samples demonstrated elevated expression of the osteolytic factor IL-6 in ever-smokers with bone metastasis among lung cancer patients. Our cellular experiments revealed that benzo[α]pyrene (B[α]P) and cigarette smoke extract (CSE) promoted IL-6 production and cell migration in lung cancer. Activation of the PI3K, Akt, and NF-κB signaling pathways was involved in cigarette smoke-augmented IL-6-dependent migration. Additionally, cigarette smoke lung cancer-secreted IL-6 promoted osteoclast formation. Importantly, blocking IL-6 abolished cigarette smoke-facilitated lung cancer osteolytic bone metastasis in vivo. Our findings provide evidence that cigarette smoke is a risk factor for osteolytic bone metastasis. Thus, inhibiting IL-6 may be a valuable therapeutic strategy for managing osteolytic bone metastasis in lung cancer patients who smoke.
Keywords: Bone metastasis; Cigarette smoke; IL-6; Lung cancer; Osteoclast.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Huang CY, Fong YC, Lee CY, Chen MY, Tsai HC, Hsu HC. et al. CCL5 increases lung cancer migration via PI3K, Akt and NF-kappaB pathways. Biochem Pharmacol. 2009;77:794–803. - PubMed
-
- Daylan AEC, Miao E, Tang K, Chiu G, Cheng H. Lung Cancer in Never Smokers: Delving into Epidemiology, Genomic and Immune Landscape, Prognosis, Treatment, and Screening. Lung. 2023;201:521–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

