Nintedanib Mitigates Radiation-Induced Pulmonary Fibrosis by Suppressing Epithelial Cell Inflammatory Response and Inhibiting Fibroblast-to-Myofibroblast Transition
- PMID: 38993568
- PMCID: PMC11234214
- DOI: 10.7150/ijbs.92620
Nintedanib Mitigates Radiation-Induced Pulmonary Fibrosis by Suppressing Epithelial Cell Inflammatory Response and Inhibiting Fibroblast-to-Myofibroblast Transition
Abstract
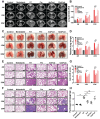
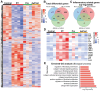
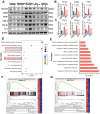
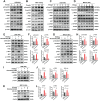
Radiation-induced pulmonary fibrosis (RIPF) represents a serious complication observed in individuals undergoing thoracic radiation therapy. Currently, effective interventions for RIPF are unavailable. Prior research has demonstrated that nintedanib, a Food and Drug Administration (FDA)-approved anti-fibrotic agent for idiopathic pulmonary fibrosis, exerts therapeutic effects on chronic fibrosing interstitial lung disease. This research aimed to investigate the anti-fibrotic influences of nintedanib on RIPF and reveal the fundamental mechanisms. To assess its therapeutic impact, a mouse model of RIPF was established. The process involved nintedanib administration at various time points, both prior to and following thoracic radiation. In the RIPF mouse model, an assessment was conducted on survival rates, body weight, computed tomography features, histological parameters, and changes in gene expression. In vitro experiments were performed to discover the mechanism underlying the therapeutic impact of nintedanib on RIPF. Treatment with nintedanib, administered either two days prior or four weeks after thoracic radiation, significantly alleviated lung pathological changes, suppressed collagen deposition, and improved the overall health status of the mice. Additionally, nintedanib demonstrated significant mitigation of radiation-induced inflammatory responses in epithelial cells by inhibiting the PI3K/AKT and MAPK signaling pathways. Furthermore, nintedanib substantially inhibited fibroblast-to-myofibroblast transition by suppressing the TGF-β/Smad and PI3K/AKT/mTOR signaling pathways. These findings suggest that nintedanib exerts preventive and therapeutic effects on RIPF by modulating multiple targets instead of a single anti-fibrotic pathway and encourage the further clinical trials to determine the efficacy of nintedanib in patients with RIPF.
Keywords: Epithelial cells; Fibroblast-to-myofibroblast transition.; Inflammatory response; Nintedanib; Radiation-induced pulmonary fibrosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Aguado-Barrera ME, Sosa-Fajardo P, Gomez-Caamano A, Taboada-Valladares B, Counago F, Lopez-Guerra JL. et al. Radiogenomics in lung cancer: Where are we? Lung Cancer. 2023;176:56–74. - PubMed
-
- Xia C, Shi W, Zhang Y, Ding L, Gao L, Wang Q. et al. Prevention and treatment of radiation-induced lung injury. Future Med Chem. 2020;12:2161–73. - PubMed
-
- Marks LB, Yu X, Vujaskovic Z, Small W Jr, Folz R, Anscher MS. Radiation-induced lung injury. Semin Radiat Oncol. 2003;13:333–45. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

