Bone or Tooth dentin: The TGF-β signaling is the key
- PMID: 38993575
- PMCID: PMC11234226
- DOI: 10.7150/ijbs.97206
Bone or Tooth dentin: The TGF-β signaling is the key
Abstract
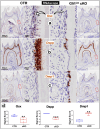
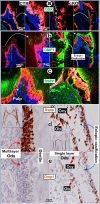
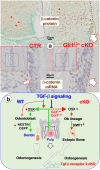
To investigate the cell linkage between tooth dentin and bones, we studied TGF-β roles during postnatal dentin development using TGF-β receptor 2 (Tgfβr2) cKO models and cell lineage tracing approaches. Micro-CT showed that the early Tgfβr2 cKO exhibit short roots and thin root dentin (n = 4; p<0.01), a switch from multilayer pre-odontoblasts/odontoblasts to a single-layer of bone-like cells with a significant loss of ~85% of dentinal tubules (n = 4; p<0.01), and a matrix shift from dentin to bone. Mechanistic studies revealed a statistically significant decrease in odontogenic markers, and a sharp increase in bone markers. The late Tgfβr2 cKO teeth displayed losses of odontoblast polarity, a significant reduction in crown dentin volume, and the onset of massive bone-like structures in the crown pulp with high expression levels of bone markers and low levels of dentin markers. We thus concluded that bones and tooth dentin are in the same evolutionary linkage in which TGF-β signaling defines the odontogenic fate of dental mesenchymal cells and odontoblasts. This finding also raises the possibility of switching the pulp odontogenic to the osteogenic feature of pulp cells via a local manipulation of gene programs in future treatment of tooth fractures.
Keywords: 3.2 kb Col1-CreERT2; Cell lineage tracing; Gli1-CreERT2; cell fate switch; evolution; tooth development.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Disruption of Tgfbr2 in odontoblasts leads to aberrant pulp calcification.J Dent Res. 2015 Jun;94(6):828-35. doi: 10.1177/0022034515577427. Epub 2015 Mar 12. J Dent Res. 2015. PMID: 25818583
-
Nuclear factor I-C is essential for odontogenic cell proliferation and odontoblast differentiation during tooth root development.J Biol Chem. 2009 Jun 19;284(25):17293-17303. doi: 10.1074/jbc.M109.009084. Epub 2009 Apr 22. J Biol Chem. 2009. PMID: 19386589 Free PMC article.
-
Axin2-CreERT2 mediated knockout of bone morphogenetic protein receptor type 1A caused abnormal secondary dentine and altered cell fate of Axin2-expressing odontogenic cells.Int Endod J. 2023 Aug;56(8):1000-1010. doi: 10.1111/iej.13933. Epub 2023 May 22. Int Endod J. 2023. PMID: 37191048
-
Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex.Congenit Anom (Kyoto). 2016 Jul;56(4):144-53. doi: 10.1111/cga.12169. Congenit Anom (Kyoto). 2016. PMID: 27131345 Review.
-
Molecular regulation of odontoblast activity under dentin injury.Adv Dent Res. 2001 Aug;15:46-50. doi: 10.1177/08959374010150011201. Adv Dent Res. 2001. PMID: 12640739 Review.
Cited by
-
Membrana preformativa: Unveiling the unexplored facets of dental development.J Oral Biol Craniofac Res. 2025 Jan-Feb;15(1):84-87. doi: 10.1016/j.jobcr.2024.12.010. Epub 2024 Dec 21. J Oral Biol Craniofac Res. 2025. PMID: 39810838 Free PMC article.
References
-
- Zhang X, Shi C, Zhao H, Zhou Y, Hu Y, Yan G. et al. Distinctive role of ACVR1 in dentin formation: requirement for dentin thickness in molars and prevention of osteodentin formation in incisors of mice. J Mol Histol. 2019;50:43–61. - PubMed
-
- Takuma S, Yanagisawa T, Lin WL. Ultrastructural and microanalytical aspects of developing osteodentin in rat incisors. Calcif Tissue Res. 1977;24:215–22. - PubMed
-
- Holmstedt JO, McClugage SG Jr, Clark JS, Guevara MJ. Osteodentin formation in continuously erupting teeth of guinea pigs. J Dent Res. 1977;56:1569–76. - PubMed
-
- Karim AC, Eddy EL. A light and electron microscopic study of osteodentin formation in the rat incisor after adriamycin administration. Am J Anat. 1984;169:207–19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

