Salvianolic acid B inhibits thrombosis and directly blocks the thrombin catalytic site
- PMID: 38993621
- PMCID: PMC11238050
- DOI: 10.1016/j.rpth.2024.102443
Salvianolic acid B inhibits thrombosis and directly blocks the thrombin catalytic site
Abstract
Background: Salvianolic acid B (SAB) is a major component of Salvia miltiorrhiza root (Danshen), widely used in East/Southeast Asia for centuries to treat cardiovascular diseases. Danshen depside salt, 85% of which is made up of SAB, is approved in China to treat chronic angina. Although clinical observations suggest that Danshen extracts inhibited arterial and venous thrombosis, the exact mechanism has not been adequately elucidated.
Objective: To delineate the antithrombotic mechanisms of SAB.
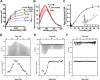
Methods: We applied platelet aggregation and coagulation assays, perfusion chambers, and intravital microscopy models. The inhibition kinetics and binding affinity of SAB to thrombin are measured by thrombin enzymatic assays, intrinsic fluorescence spectrophotometry, and isothermal titration calorimetry. We used molecular in silico docking models to predict the interactions of SAB with thrombin.
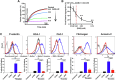
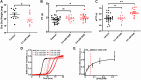
Results: SAB dose-dependently inhibited platelet activation and aggregation induced by thrombin. SAB also reduced platelet aggregation induced by adenosine diphosphate and collagen. SAB attenuated blood coagulation by modifying fibrin network structures and significantly decreased thrombus formation in mouse cremaster arterioles and perfusion chambers. The direct SAB-thrombin interaction was confirmed by enzymatic assays, intrinsic fluorescence spectrophotometry, and isothermal titration calorimetry. Interestingly, SAB shares key structural similarities with the trisubstituted benzimidazole class of thrombin inhibitors, such as dabigatran. Molecular docking models predicted the binding of SAB to the thrombin active site.
Conclusion: Our data established SAB as the first herb-derived direct thrombin catalytic site inhibitor, suppressing thrombosis through both thrombin-dependent and thrombin-independent pathways. Purified SAB may be a cost-effective agent for treating arterial and deep vein thrombosis.
Keywords: blood coagulation; platelet; salvianolic acid B (SAB); thrombin; thrombosis.
© 2024 The Authors.
Figures



References
-
- Wang Y., Andrews M., Yang Y., Lang S., Jin J.W., Cameron-Vendrig A., et al. Platelets in thrombosis and hemostasis: old topic with new mechanisms. Cardiovasc Hematol Disord Drug Targets. 2012;12:126–132. - PubMed
-
- Xu X.R., Gallant R.C., Ni H. Platelets, immune-mediated thrombocytopenias, and fetal hemorrhage. Thromb Res. 2016;141:S76–S79. - PubMed
-
- Stroncek D.F., Rebulla P. Platelet transfusions. Lancet. 2007;370:427–438. - PubMed
LinkOut - more resources
Full Text Sources

