Modeling hepatitis A epidemiological profiles and estimating the pediatric vaccination threshold in the Russian Federation
- PMID: 38993707
- PMCID: PMC11236541
- DOI: 10.3389/fpubh.2024.1371996
Modeling hepatitis A epidemiological profiles and estimating the pediatric vaccination threshold in the Russian Federation
Abstract
Background: To combat the hesitancy towards implementing a hepatitis A universal mass vaccination (UMV) strategy and to provide healthcare authorities with a comprehensive analysis of the potential outcomes and benefits of the implementation of such a vaccination program, we projected HAV seroprevalence and incidence rates in the total population of the Russian Federation and estimated the pediatric vaccination threshold required to achieve an incidence level of less than 1 case per 100,000 using a new mathematical model.
Methods: A dynamic age-structured SEIRV (susceptible-exposed-infectious-recovered-vaccinated) compartmental model was developed and calibrated using demographic, seroprevalence, vaccination, and epidemiological data from different regions of the Russian Federation. This model was used to project various epidemiological measures.
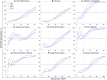
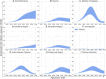
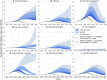
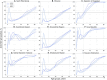
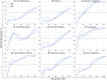
Results: The projected national average age at the midpoint of population immunity increases from 40 years old in 2020 to 50 years old in 2036 and is shifted even further to the age of 70 years in some regions of the country. An increase of varying magnitude in the incidence of symptomatic HAV infections is predicted for all study regions and for the Russian Federation as a whole between 2028 and 2032, if the HAV vaccination coverage level remains at the level of 2022. The national average vaccination coverage level required to achieve a symptomatic HAV incidence rate below 1 case per 100,000 by 2032 was calculated to be 69.8% if children aged 1-6 years are vaccinated following the implementation of a UMV program or 34.8% if immunization is expanded to children aged 1-17 years.
Conclusion: The developed model provides insights into a further decline of herd immunity to HAV against the background of ongoing viral transmission. The current favorable situation regarding hepatitis A morbidity is projected to be replaced by an increase in incidence rates if vaccination coverage remains at the current levels. The obtained results support the introduction of a hepatitis A UMV strategy in the Russian Federation.
Keywords: HAV; hepatitis A; hepatitis A vaccination; herd immunity; mathematical model.
Copyright © 2024 Taratorkin, Karlsen, Kyuregyan, Lopatukhina, Khankishiyev, Manuylov, Akimkin and Mikhailov.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- World Health Organization . WHO immunological basis for immunization series, module 18: Hepatitis A update. Geneva, Switzerland: WHO; (2019).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

