Coordinated regulation of osmotic imbalance by c-di-AMP shapes ß-lactam tolerance in Group B Streptococcus
- PMID: 38993744
- PMCID: PMC11238645
- DOI: 10.1093/femsml/uqae014
Coordinated regulation of osmotic imbalance by c-di-AMP shapes ß-lactam tolerance in Group B Streptococcus
Abstract
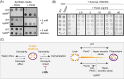
Streptococcus agalactiae is among the few pathogens that have not developed resistance to ß-lactam antibiotics despite decades of clinical use. The molecular basis of this long-lasting susceptibility has not been investigated, and it is not known whether specific mechanisms constrain the emergence of resistance. In this study, we first report ß-lactam tolerance due to the inactivation of the c-di-AMP phosphodiesterase GdpP. Mechanistically, tolerance depends on antagonistic regulation by the repressor BusR, which is activated by c-di-AMP and negatively regulates ß-lactam susceptibility through the BusAB osmolyte transporter and the AmaP/Asp23/GlsB cell envelope stress complex. The BusR transcriptional response is synergistic with the simultaneous allosteric inhibition of potassium and osmolyte transporters by c-di-AMP, which individually contribute to low-level ß-lactam tolerance. Genome-wide transposon mutagenesis confirms the role of GdpP and highlights functional interactions between a lysozyme-like hydrolase, the KhpAB RNA chaperone and the protein S immunomodulator in the response of GBS to ß-lactam. Overall, we demonstrate that c-di-AMP acts as a turgor pressure rheostat, coordinating an integrated response at the transcriptional and post-translational levels to cell wall weakening caused by ß-lactam activity, and reveal additional mechanisms that could foster resistance.
Keywords: Streptococcus; antibiotic; cell wall; nucleotide signaling; osmolytes; turgor pressure.
© The Author(s) 2024. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Ba X, Kalmar L, Hadjirin NF et al. Truncation of GdpP mediates β-lactam resistance in clinical isolates of Staphylococcus aureus. J Antimicrob Chemother. 2019;74:1182–91. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
