Highly Enantiomerically Enriched Secondary Alcohols via Epoxide Hydrogenolysis
- PMID: 38993820
- PMCID: PMC11234370
- DOI: 10.1021/acs.organomet.4c00214
Highly Enantiomerically Enriched Secondary Alcohols via Epoxide Hydrogenolysis
Abstract
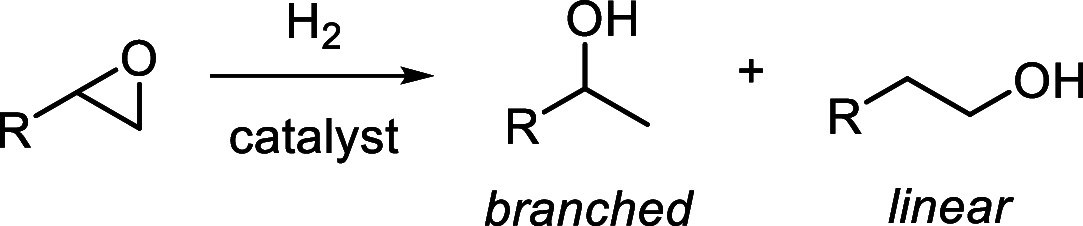
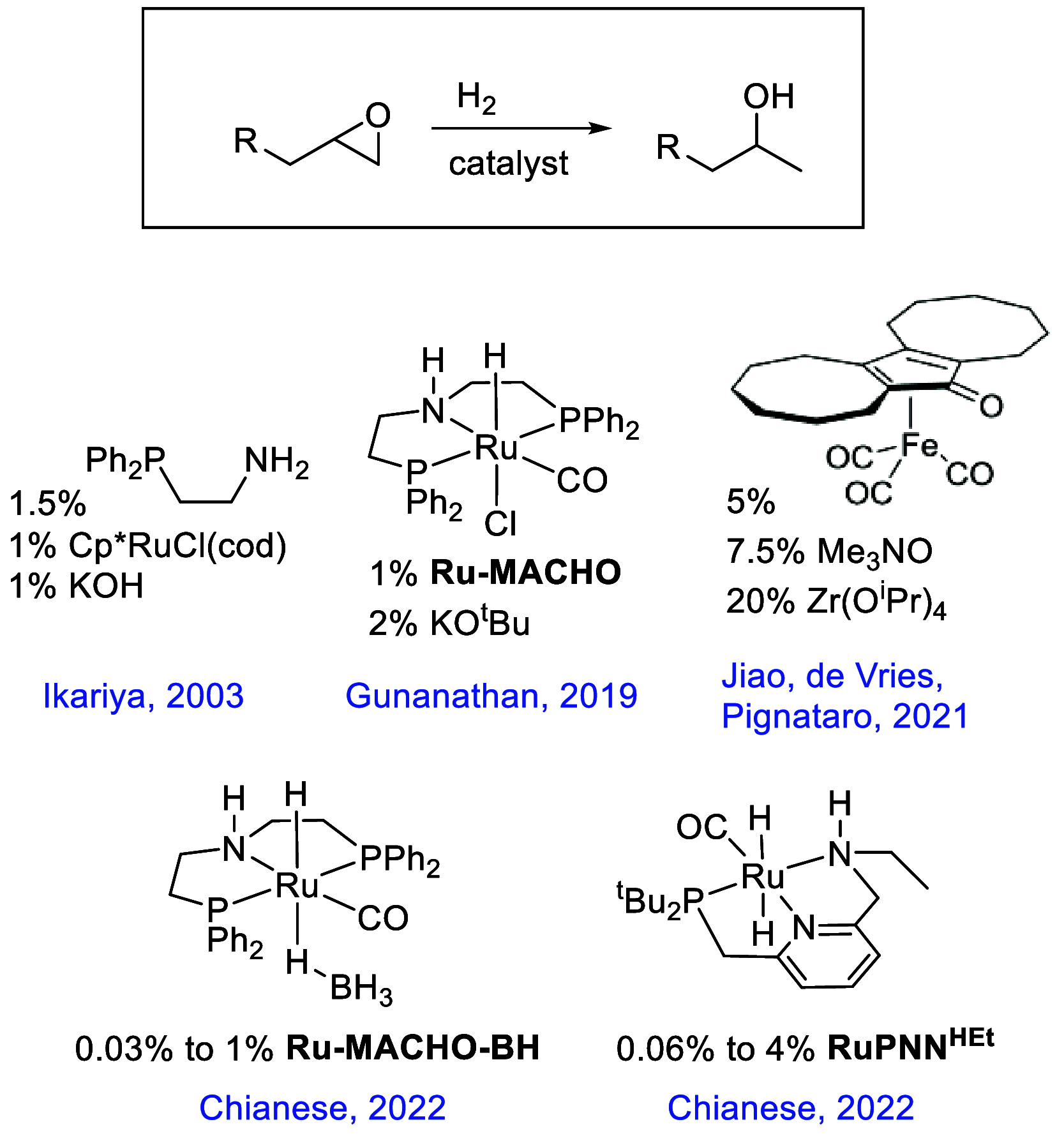
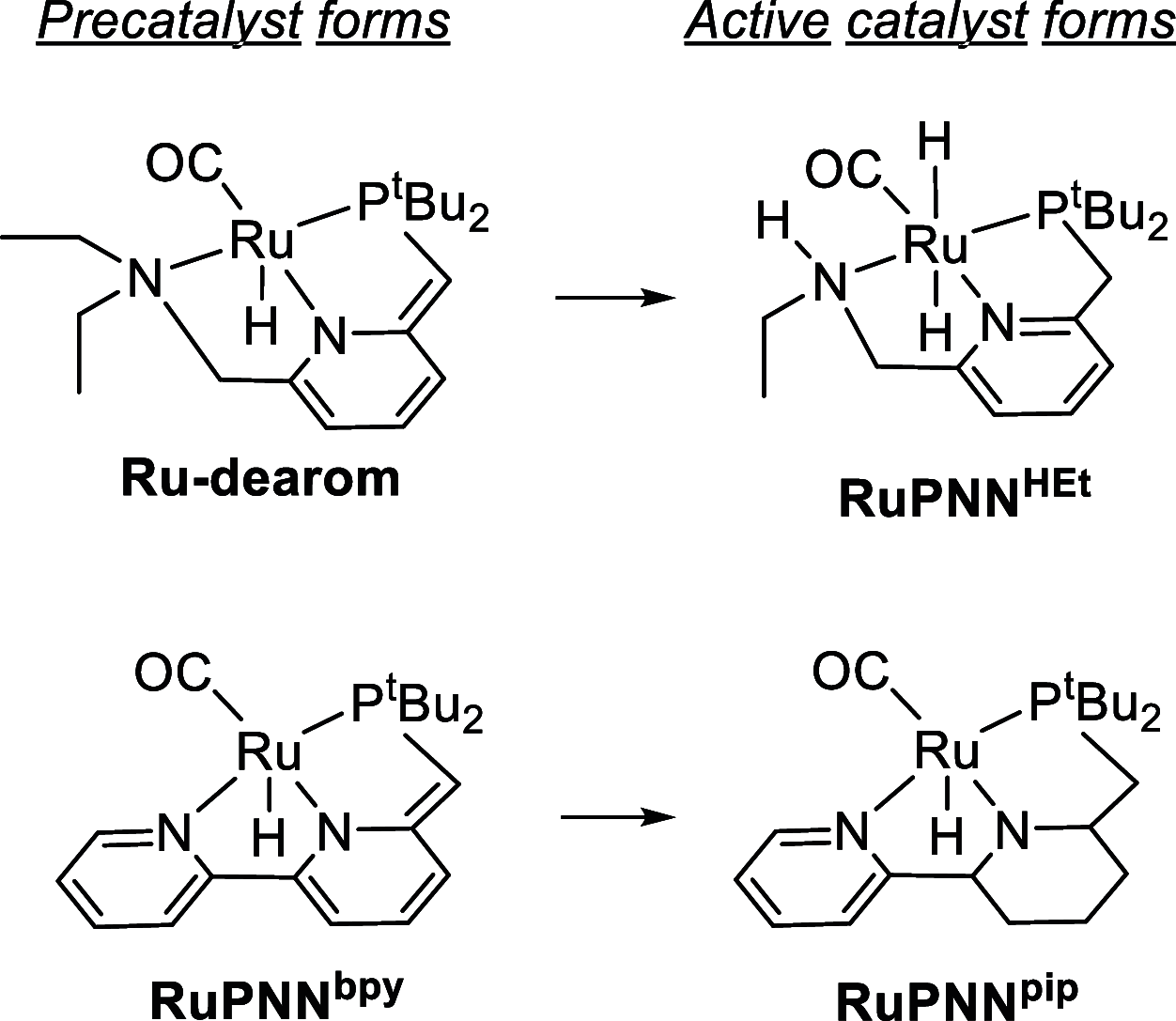
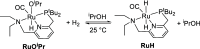
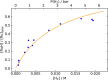
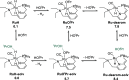
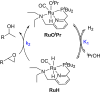
In this article, we report the development of ruthenium-catalyzed hydrogenolysis of epoxides to selectively give the branched (Markovnikov) alcohol products. In contrast to previously reported catalysts, the use of Milstein's PNN-pincer-ruthenium complex at room temperature allows the conversion of enantiomerically enriched epoxides to secondary alcohols without racemization of the product. The catalyst is effective for a range of aryl epoxides, alkyl epoxides, and glycidyl ethers and is the first homogeneous system to selectively promote hydrogenolysis of glycidol to 1,2-propanediol, without loss of enantiomeric purity. A detailed mechanistic study was conducted, including experimental observations of catalyst speciation under catalytically relevant conditions, comprehensive kinetic characterization of the catalytic reaction, and computational analysis via density functional theory. Heterolytic hydrogen cleavage is mediated by the ruthenium center and exogenous alkoxide base. Epoxide ring opening occurs through an opposite-side attack of the ruthenium hydride on the less-hindered epoxide carbon, giving the branched alcohol product selectively.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

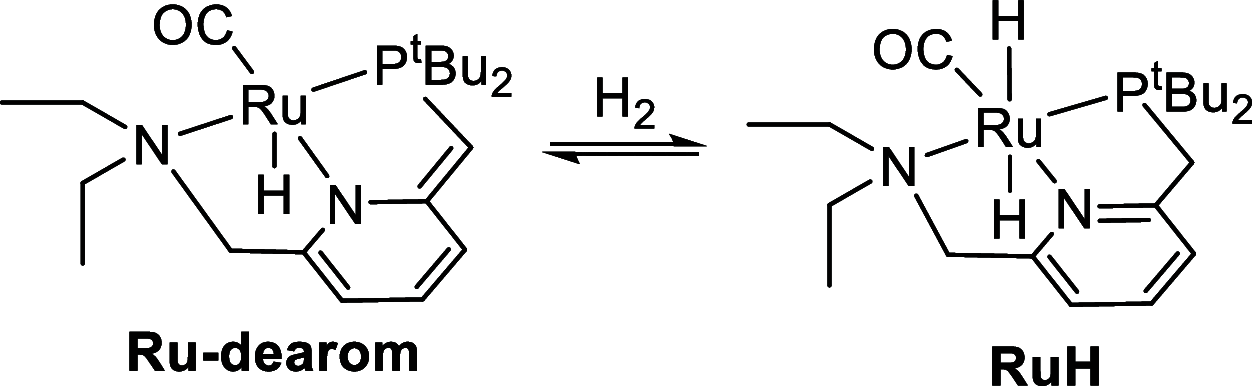


Similar articles
-
The Mechanism of Markovnikov-Selective Epoxide Hydrogenolysis Catalyzed by Ruthenium PNN and PNP Pincer Complexes.Organometallics. 2023 Feb 27;42(5):347-356. doi: 10.1021/acs.organomet.2c00503. eCollection 2023 Mar 13. Organometallics. 2023. PMID: 36937786 Free PMC article.
-
The key role of the latent N-H group in Milstein's catalyst for ester hydrogenation.Chem Sci. 2021 May 24;12(24):8477-8492. doi: 10.1039/d1sc00703c. eCollection 2021 Jun 23. Chem Sci. 2021. PMID: 35355805 Free PMC article.
-
Combined ruthenium(II) and lipase catalysis for efficient dynamic kinetic resolution of secondary alcohols. Insight into the racemization mechanism.J Am Chem Soc. 2005 Jun 22;127(24):8817-25. doi: 10.1021/ja051576x. J Am Chem Soc. 2005. PMID: 15954789
-
Effect of Tungsten Species on Selective Hydrogenolysis of Glycerol to 1,3-Propanediol.ChemSusChem. 2021 Jan 21;14(2):569-581. doi: 10.1002/cssc.202002405. Epub 2020 Dec 4. ChemSusChem. 2021. PMID: 33219614 Review.
-
Recent advances in osmium-catalyzed hydrogenation and dehydrogenation reactions.Acc Chem Res. 2015 Feb 17;48(2):363-79. doi: 10.1021/ar5003818. Epub 2015 Feb 4. Acc Chem Res. 2015. PMID: 25650714 Review.
References
-
- Ito M.; Hirakawa M.; Osaku A.; Ikariya T. Highly Efficient Chemoselective Hydrogenolysis of Epoxides Catalyzed by a (Η5-C5(CH3)5)Ru Complex Bearing a 2-(Diphenylphosphino)Ethylamine Ligand. Organometallics 2003, 22, 4190–4192. 10.1021/om034006j. - DOI
-
- Rainsberry A. N.; Sage J. G.; Scheuermann M. L. Iridium-Promoted Conversion of Terminal Epoxides to Primary Alcohols under Acidic Conditions Using Hydrogen. Catal. Sci. Technol. 2019, 9, 3020–3022. 10.1039/C9CY00791A. - DOI
-
- Gitnes R. M.; Wang M.; Bao Y.; Scheuermann M. L. In Situ Generation of Catalytically Relevant Nanoparticles from a Molecular Pincer Iridium Precatalyst During Polyol Deoxygenation. ACS Catal. 2021, 11, 495–501. 10.1021/acscatal.0c03180. - DOI
-
- Liu W.; Li W.; Spannenberg A.; Junge K.; Beller M. Iron-Catalysed Regioselective Hydrogenation of Terminal Epoxides to Alcohols under Mild Conditions. Nature Catalysis 2019, 2, 523–528. 10.1038/s41929-019-0286-7. - DOI
LinkOut - more resources
Full Text Sources
