Oxygen carriers affect kidney immunogenicity during ex-vivo machine perfusion
- PMID: 38993849
- PMCID: PMC11235266
- DOI: 10.3389/frtra.2023.1183908
Oxygen carriers affect kidney immunogenicity during ex-vivo machine perfusion
Abstract
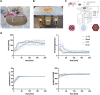
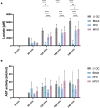
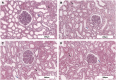
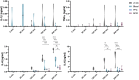
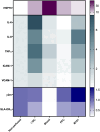
Normothermic ex-vivo machine perfusion provides a powerful tool to improve donor kidney preservation and a route for the delivery of pharmacological or gene therapeutic interventions prior to transplantation. However, perfusion at normothermic temperatures requires adequate tissue oxygenation to meet the physiological metabolic demand. For this purpose, the addition of appropriate oxygen carriers (OCs) to the perfusion solution is essential to ensure a sufficient oxygen supply and reduce the risk for tissue injury due to hypoxia. It is crucial that the selected OCs preserve the integrity and low immunogenicity of the graft. In this study, the effect of two OCs on the organ's integrity and immunogenicity was evaluated. Porcine kidneys were perfused ex-vivo for four hours using perfusion solutions supplemented with red blood cells (RBCs) as conventional OC, perfluorocarbon (PFC)-based OC, or Hemarina-M101 (M101), a lugworm hemoglobin-based OC named HEMO2life®, recently approved in Europe (i.e., CE obtained in October 2022). Perfusions with all OCs led to decreased lactate levels. Additionally, none of the OCs negatively affected renal morphology as determined by histological analyses. Remarkably, all OCs improved the perfusion solution by reducing the expression of pro-inflammatory mediators (IL-6, IL-8, TNFα) and adhesion molecules (ICAM-1) on both transcript and protein level, suggesting a beneficial effect of the OCs in maintaining the low immunogenicity of the graft. Thus, PFC-based OCs and M101 may constitute a promising alternative to RBCs during normothermic ex-vivo kidney perfusion.
Keywords: HEMO2Life; hemarina-M101; kidney preservation; normothermic ex-vivo machine perfusion; organ immunogenicity; oxygen carriers; perfluorocarbon-based oxygen carriers; transplantation.
© 2023 Rother, Horgby, Schmalkuche, Burgmann, Nocke, Jägers, Schmitz, Bräsen, Cantore, ZLC, Ferenz, Blasczyk and Figueiredo.
Conflict of interest statement
The authors JHB and CF declared that they were editorial board members of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

