Recipient TIM4 signaling regulates ischemia reperfusion-induced ER stress and metabolic responses in liver transplantation: from mouse-to-human
- PMID: 38993869
- PMCID: PMC11235257
- DOI: 10.3389/frtra.2023.1176384
Recipient TIM4 signaling regulates ischemia reperfusion-induced ER stress and metabolic responses in liver transplantation: from mouse-to-human
Abstract
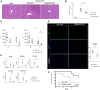
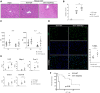
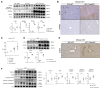
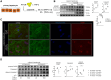
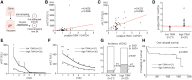
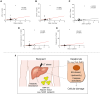
T-cell immunoglobulin and mucin (Tim)4 is expressed on APCs, including macrophages, as one of the main amplifiers in the mechanism of liver ischemia-reperfusion injury (IRI) following orthotopic liver transplantation (OLT). Though donor Tim4 selectively expressed on Kupffer cells serves as a checkpoint regulator of innate immune-driven IRI cascades, its role on cells outside the OLT remains unclear. To dissect the role of donor vs. recipient-specific Tim4 signaling in IR-induced stress and hepatocellular function, we employed a murine OLT model utilizing Tim4-knockout (KO) mice as either donor or recipient (WT → WT, WT → Tim4-KO, Tim4-KO → WT). In the experimental arm, disruption of donor Tim4 attenuated IRI-OLT damage, while recipient Tim4-null mutation aggravated hepatic IRI concomitant with disturbed lipid metabolism, enhanced endoplasmic reticulum stress, and activated pro-apoptotic signaling in the grafts. In the in vitro study, murine hepatocytes co-cultured with Tim4-null adipose tissue showed enhanced C/EBP homologous protein (CHOP) expression pattern and susceptibility to hepatocellular death accompanied by activated caspase cascade in response to TNF-α stimulation. In the clinical arm, liver grafts from forty-one transplant patients with enhanced TIM4 expression showed higher body mass index, augmented hepatic endoplasmic reticulum stress, enhanced pro-apoptotic markers, upregulated innate/adaptive immune responses, exacerbated hepatocellular damage, and inferior graft survival. In conclusion, although TIM4 is considered a principal villain in peri-transplant early tissue injury, recipient TIM4 signaling may serve as a savior of IR-triggered metabolic stress in mouse and human OLT recipients.
Keywords: endoplasmic reticulum stress; ischemia-reperfusion injury; liver transplantation; orthotopic liver tranplantation; t-cell immunoglobulin and mucin domain containing 4.
© 2023 Hirao, Kageyama, Nakamura, Kadono, Kojima, Siyuan, Farmer, Kaldas, Dery and Kupiec-Weglinski.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Kupiec-Weglinski JW. Grand challenges in organ transplantation. Front Transpl. (2022) 1:897679. 10.3389/frtra.2022.897679 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

