Treatment of steroid-refractory graft versus host disease in children
- PMID: 38993897
- PMCID: PMC11235274
- DOI: 10.3389/frtra.2023.1251112
Treatment of steroid-refractory graft versus host disease in children
Abstract
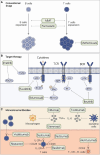
Systemic steroids are still the first-line approach in acute graft-versus-host disease (aGvHD), and the backbone of chronic GvHD management. Refractoriness to steroid represent a major cause of morbidity and non-relapse mortality after hematopoietic stem cell transplantation (HSCT). In both backgrounds, several second-line immunosuppressive agents have been tested with variable results in terms of efficacy and toxicity. Solid evidence regarding these approaches is still lacking in the pediatric setting where results are mainly derived from adult experiences. Furthermore, the number of treated patients is limited and the incidence of acute and chronic GvHD is lower, resulting in a very heterogeneous approach to this complication by pediatric hematologists. Some conventional therapies and anti-cytokine monoclonal antibodies used in the adult setting have been evaluated in children. In recent years, the increasing understanding of the biological mechanisms underpinning the pathogenesis of GvHD justified the efforts toward the adoption of targeted therapies and non-pharmacologic approaches, with higher response rates and lower immunosuppressive effects. Moreover, many questions regarding the precise timing and setting in which to integrate these new approaches remain unanswered. This Review aims to critically explore the current evidence regarding novel approaches to treat SR-GvHD in pediatric HSCT recipients.
Keywords: GvHD; children; pediatric HSCT; ruxolitinib; steroid-refractory.
© 2023 Gottardi, Leardini, Muratore, Baccelli, Cerasi, Venturelli, Zanaroli, Belotti, Prete and Masetti.
Conflict of interest statement
The author (RM) declared that he was an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials