Liquid biopsy for non-invasive monitoring of patients with kidney transplants
- PMID: 38993899
- PMCID: PMC11235308
- DOI: 10.3389/frtra.2023.1148725
Liquid biopsy for non-invasive monitoring of patients with kidney transplants
Abstract
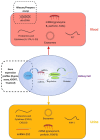
The current tools for diagnosing and monitoring native kidney diseases as well as allograft rejection in transplant patients are suboptimal. Creatinine and proteinuria are non-specific and poorly sensitive markers of injury. Tissue biopsies are invasive and carry potential complications. In this article, we overview the different techniques of liquid biopsy and discuss their potential to improve patients' kidney health. Several diagnostic, predictive, and prognostic biomarkers have been identified with the ability to detect and monitor the activity of native kidney diseases as well as early and chronic allograft rejection, such as donor-derived cell-free DNA, exosomes, messenger RNA/microsomal RNA, proteomics, and so on. While the results are encouraging, additional research is still needed as no biomarker appears to be perfect for a routine application in clinical practice. Despite promising advancements in biomarkers, the most important issue is the lack of standardized pre-analytical criteria. Large validation studies and uniformed standard operating procedures are required to move the findings from bench to bedside. Establishing consortia such as the Liquid Biopsy Consortium for Kidney Diseases can help expedite the research process, allow large studies to establish standardized procedures, and improve the management and outcomes of kidney diseases and of kidney transplant recipients.
Keywords: biomarkers; cytokines; ddcfDNA; exosomes; kidney diseases; kidney transplantation; liquid biopsy; proteomics.
© 2023 Nassar, Cashman, Rao, Dagher, O'Brien, Afif, Cravedi and Azzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors JRA and PC declared that they were editorial board members of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision. This had no impact on the peer review process and the final decision. The reviewer AA declared a shared parent affiliation with the authors AN, KC, SR, MD, COB, JA, and JRA to the handling editor at the time of review.
Figures
References
-
- Kidney Disease Statistics for the United States | NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases. Available at: https://www.niddk.nih.gov/health-information/health-statistics/kidney-di... (Accessed December 19, 2022).
Publication types
LinkOut - more resources
Full Text Sources

