Farnesyltransferase-inhibitors exert in vitro immunosuppressive capacity by inhibiting human B-cells
- PMID: 38993912
- PMCID: PMC11235315
- DOI: 10.3389/frtra.2023.1233322
Farnesyltransferase-inhibitors exert in vitro immunosuppressive capacity by inhibiting human B-cells
Abstract
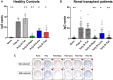
Objectives: Farnesyltransferase inhibitors (FTI), which inhibit the prenylation of Ras GTPases, were developed as anti-cancer drugs. As additional target proteins for prenylation were identified in the past, it is likely that FTI have potential value for therapeutic purposes beyond cancer. The effect of FTI on B-cells remains unclear. To address this issue, we investigated the effects of in vitro FTI treatment on effector and regulatory B-cells in healthy controls and renal transplant patients.
Methods: For this purpose, B-cells were isolated from the peripheral blood of healthy controls and renal transplant patients. Purified B-cells were stimulated via Toll-like-receptor 9 (TLR-9) in the presence or absence of FTI. Regulatory functions, such as IL-10 and Granzyme B (GrB) secretion, were assessed by flow cytometry. In addition, effector B-cell functions, such as plasma cell formation and IgG secretion, were studied.
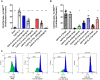
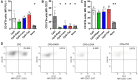
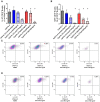
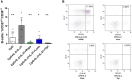
Results: The two FTI Lonafarnib and tipifarnib both suppressed TLR-9-induced B-cell proliferation. Maturation of IL-10 producing B-cells was suppressed by FTI at high concentrations as well as induction of GrB-secreting B-cells. Plasma blast formation and IgG secretion were potently suppressed by FTI. Moreover, purified B-cells from immunosuppressed renal transplant patients were also susceptible to FTI-induced suppression of effector functions, evidenced by diminished IgG secretion.
Conclusion: FTI suppress in vitro B-cell proliferation and plasma cell formation while partially preserving IL-10 as well as GrB production of B-cells. Thus, FTI may have immunosuppressive capacity encouraging further studies to investigate the potential immunomodulatory value of this agent.
Keywords: B-cells; farnesyltransferase inhibitors; humoral rejection; plasma cells; renal transplantation.
© 2023 Xu, Dolff, Mülling, Bachmann, Dai, Lindemann, Sun, Witzke, Kribben and Wilde.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors BW declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Chloroquine Suppresses Effector B-Cell Functions and Has Differential Impact on Regulatory B-Cell Subsets.Front Immunol. 2022 Feb 8;13:818704. doi: 10.3389/fimmu.2022.818704. eCollection 2022. Front Immunol. 2022. PMID: 35211119 Free PMC article.
-
Farnesyltransferase inhibitor FTI-277 inhibits PD-L1 expression on septic spleen lymphocytes and promotes spleen lymphocyte activation.Clin Exp Immunol. 2017 Oct;190(1):8-18. doi: 10.1111/cei.12995. Epub 2017 Jul 14. Clin Exp Immunol. 2017. PMID: 28556912 Free PMC article.
-
Combining prenylation inhibitors causes synergistic cytotoxicity, apoptosis and disruption of RAS-to-MAP kinase signalling in multiple myeloma cells.Br J Haematol. 2005 Sep;130(6):912-25. doi: 10.1111/j.1365-2141.2005.05696.x. Br J Haematol. 2005. PMID: 16156861
-
Farnesyltransferase inhibitor as anticancer agent.Mini Rev Med Chem. 2009 Jun;9(6):638-52. doi: 10.2174/138955709788452702. Mini Rev Med Chem. 2009. PMID: 19519490 Review.
-
Evolving therapies: farnesyltransferase inhibitors.Curr Oncol Rep. 2002 Jan;4(1):29-36. doi: 10.1007/s11912-002-0045-8. Curr Oncol Rep. 2002. PMID: 11734111 Review.
Cited by
-
Anti-PD1 prolongs the response of PI3K and farnesyl transferase inhibition in HRAS- and PIK3CA-mutant head and neck cancers.Neoplasia. 2025 May;63:101157. doi: 10.1016/j.neo.2025.101157. Epub 2025 Mar 20. Neoplasia. 2025. PMID: 40117718 Free PMC article.
References
-
- Karp JE, Lancet JE, Kaufmann SH, End DW, Wright JJ, Bol K, et al. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase 1 clinical-laboratory correlative trial. Blood. (2001) 97(11):3361–9. 10.1182/blood.v97.11.3361 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

