Biomarker-driven molecular imaging probes in radiotherapy
- PMID: 38994026
- PMCID: PMC11234278
- DOI: 10.7150/thno.97768
Biomarker-driven molecular imaging probes in radiotherapy
Abstract
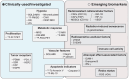
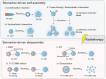
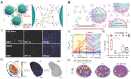
Background: Biomarker-driven molecular imaging has emerged as an integral part of cancer precision radiotherapy. The use of molecular imaging probes, including nanoprobes, have been explored in radiotherapy imaging to precisely and noninvasively monitor spatiotemporal distribution of biomarkers, potentially revealing tumor-killing mechanisms and therapy-induced adverse effects during radiation treatment. Methods: We summarized literature reports from preclinical studies and clinical trials, which cover two main parts: 1) Clinically-investigated and emerging imaging biomarkers associated with radiotherapy, and 2) instrumental roles, functions, and activatable mechanisms of molecular imaging probes in the radiotherapy workflow. In addition, reflection and future perspectives are proposed. Results: Numerous imaging biomarkers have been continuously explored in decades, while few of them have been successfully validated for their correlation with radiotherapeutic outcomes and/or radiation-induced toxicities. Meanwhile, activatable molecular imaging probes towards the emerging biomarkers have exhibited to be promising in animal or small-scale human studies for precision radiotherapy. Conclusion: Biomarker-driven molecular imaging probes are essential for precision radiotherapy. Despite very inspiring preliminary results, validation of imaging biomarkers and rational design strategies of probes await robust and extensive investigations. Especially, the correlation between imaging biomarkers and radiotherapeutic outcomes/toxicities should be established through multi-center collaboration involving a large cohort of patients.
Keywords: biomarker; imaging probe; molecular imaging; nanoparticle; radiotherapy.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Preclinical in vivo cancer, straightway to patients?Q J Nucl Med Mol Imaging. 2017 Jun;61(2):145-152. doi: 10.23736/S1824-4785.17.02974-0. Q J Nucl Med Mol Imaging. 2017. PMID: 28347131 Review.
-
Activatable Molecular Probes for Second Near-Infrared Fluorescence, Chemiluminescence, and Photoacoustic Imaging.Angew Chem Int Ed Engl. 2020 Jul 13;59(29):11717-11731. doi: 10.1002/anie.202001783. Epub 2020 Apr 1. Angew Chem Int Ed Engl. 2020. PMID: 32134156 Review.
-
Protein-based tumor molecular imaging probes.Amino Acids. 2011 Nov;41(5):1013-36. doi: 10.1007/s00726-010-0545-z. Epub 2010 Mar 17. Amino Acids. 2011. PMID: 20232092 Free PMC article. Review.
-
Targeting Peptide-Based Probes for Molecular Imaging and Diagnosis.Adv Mater. 2019 Nov;31(45):e1804827. doi: 10.1002/adma.201804827. Epub 2018 Dec 7. Adv Mater. 2019. PMID: 30537222 Review.
-
Generation of hydroxyl radical-activatable ratiometric near-infrared bimodal probes for early monitoring of tumor response to therapy.Nat Commun. 2021 Oct 22;12(1):6145. doi: 10.1038/s41467-021-26380-y. Nat Commun. 2021. PMID: 34686685 Free PMC article.
Cited by
-
An update on recent advances in fluorescent materials for fluorescence molecular imaging: a review.RSC Adv. 2025 Jun 30;15(28):22267-22284. doi: 10.1039/d5ra03102h. eCollection 2025 Jun 30. RSC Adv. 2025. PMID: 40599579 Free PMC article. Review.
-
Nanomedicine in glaucoma treatment; Current challenges and future perspectives.Mater Today Bio. 2024 Sep 4;28:101229. doi: 10.1016/j.mtbio.2024.101229. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39296355 Free PMC article. Review.
-
Copper-luteolin nanocomplexes for Mediating multifaceted regulation of oxidative stress, intestinal barrier, and gut microbiota in inflammatory bowel disease.Bioact Mater. 2024 Dec 11;46:118-133. doi: 10.1016/j.bioactmat.2024.12.004. eCollection 2025 Apr. Bioact Mater. 2024. PMID: 39760067 Free PMC article.
-
Benzo[a]phenoselenazine-based NIR photodynamic therapy for the treatment of COX-2 overexpressing cancer cells.Future Med Chem. 2025 Feb;17(4):425-434. doi: 10.1080/17568919.2025.2463878. Epub 2025 Feb 14. Future Med Chem. 2025. PMID: 39953784
-
Advancements in CRISPR/Cas systems for disease treatment.Acta Pharm Sin B. 2025 Jun;15(6):2818-2844. doi: 10.1016/j.apsb.2025.05.007. Epub 2025 May 17. Acta Pharm Sin B. 2025. PMID: 40654372 Free PMC article. Review.
References
-
- Olin AB, Hansen AE, Rasmussen JH, Ladefoged CN, Berthelsen AK, Håkansson K. et al. Feasibility of multiparametric positron emission tomography/magnetic resonance imaging as a one-stop shop for radiation therapy planning for patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2020;108:1329–38. - PubMed
-
- Vitzthum LK, Surucu M, Gensheimer MF, Kovalchuk N, Han B, Pham D. et al. BIOGUIDE-X: a first-in-human study of the performance of positron emission tomography-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2024;118:1172–80. - PubMed
-
- Michaelis LC, Ratain MJ. Measuring response in a post-RECIST world: from black and white to shades of grey. Nat Rev Cancer. 2006;6:409–14. - PubMed
-
- Li H, Luo Q, Zhang H, Ma X, Gu Z, Gong Q, Luo K. Nanomedicine embraces cancer radio-immunotherapy: mechanism, design, recent advances, and clinical translation. Chem Soc Rev. 2023;52:47–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

