Bioinspired soft-hard combined system with mild photothermal therapeutic activity promotes diabetic bone defect healing via synergetic effects of immune activation and angiogenesis
- PMID: 38994032
- PMCID: PMC11234279
- DOI: 10.7150/thno.97335
Bioinspired soft-hard combined system with mild photothermal therapeutic activity promotes diabetic bone defect healing via synergetic effects of immune activation and angiogenesis
Abstract
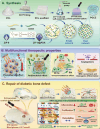
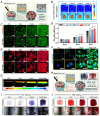
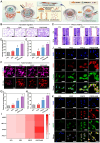
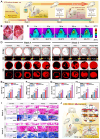
Background: The comprehensive management of diabetic bone defects remains a substantial clinical challenge due to the hostile regenerative microenvironment characterized by aggravated inflammation, excessive reactive oxygen species (ROS), bacterial infection, impaired angiogenesis, and unbalanced bone homeostasis. Thus, an advanced multifunctional therapeutic platform capable of simultaneously achieving immune regulation, bacterial elimination, and tissue regeneration is urgently designed for augmented bone regeneration under diabetic pathological milieu. Methods and Results: Herein, a photoactivated soft-hard combined scaffold system (PGCZ) was engineered by introducing polydopamine-modified zeolitic imidazolate framework-8-loaded double-network hydrogel (soft matrix component) into 3D-printed poly(ε-caprolactone) (PCL) scaffold (hard matrix component). The versatile PGCZ scaffold based on double-network hydrogel and 3D-printed PCL was thus prepared and features highly extracellular matrix-mimicking microstructure, suitable biodegradability and mechanical properties, and excellent photothermal performance, allowing long-term structural stability and mechanical support for bone regeneration. Under periodic near-infrared (NIR) irradiation, the localized photothermal effect of PGCZ triggers the on-demand release of Zn2+, which, together with repeated mild hyperthermia, collectively accelerates the proliferation and osteogenic differentiation of preosteoblasts and potently inhibits bacterial growth and biofilm formation. Additionally, the photoactivated PGCZ system also presents outstanding immunomodulatory and ROS scavenging capacities, which regulate M2 polarization of macrophages and drive functional cytokine secretion, thus leading to a pro-regenerative microenvironment in situ with enhanced vascularization. In vivo experiments further demonstrated that the PGCZ platform in conjunction with mild photothermal therapeutic activity remarkably attenuated the local inflammatory cascade, initiated endogenous stem cell recruitment and neovascularization, and orchestrated the osteoblast/osteoclast balance, ultimately accelerating diabetic bone regeneration. Conclusions: This work highlights the potential application of a photoactivated soft-hard combined system that provides long-term biophysical (mild photothermal stimulation) and biochemical (on-demand ion delivery) cues for accelerated healing of diabetic bone defects.
Keywords: 3D-printed scaffold; angiogenesis; bioactive hydrogel; diabetic bone regeneration; immune microenvironment; mild photothermal stimulation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures








Similar articles
-
Multifunctional Hydrogel with Synergistic Reactive Oxygen Species Scavenging and Macrophage Polarization-Induced Osteo-immunomodulation for Enhanced Bone Regeneration.ACS Appl Mater Interfaces. 2025 Jul 9;17(27):38985-39001. doi: 10.1021/acsami.5c08737. Epub 2025 Jun 24. ACS Appl Mater Interfaces. 2025. PMID: 40552867 Free PMC article.
-
Multifunctional injectable hydrogel system as a mild photothermal-assisted therapeutic platform for programmed regulation of inflammation and osteo-microenvironment for enhanced healing of diabetic bone defects in situ.Theranostics. 2024 Oct 28;14(18):7140-7198. doi: 10.7150/thno.102779. eCollection 2024. Theranostics. 2024. PMID: 39629118 Free PMC article.
-
Smart-Responsive Multifunctional Therapeutic System for Improved Regenerative Microenvironment and Accelerated Bone Regeneration via Mild Photothermal Therapy.Adv Sci (Weinh). 2024 Jan;11(2):e2304641. doi: 10.1002/advs.202304641. Epub 2023 Nov 7. Adv Sci (Weinh). 2024. PMID: 37933988 Free PMC article.
-
Multifunctional microneedle-mediated photothermo-gas-ion synergic therapy accelerates MRSA infacted diabetic wound healing.Mater Today Bio. 2025 May 24;32:101903. doi: 10.1016/j.mtbio.2025.101903. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40510833 Free PMC article. Review.
-
Dynamic ion-releasing biomaterials actively shape the microenvironment to enhance healing.J Trace Elem Med Biol. 2025 Jun;89:127657. doi: 10.1016/j.jtemb.2025.127657. Epub 2025 Apr 17. J Trace Elem Med Biol. 2025. PMID: 40250222 Review.
Cited by
-
Application of Light-Responsive Nanomaterials in Bone Tissue Engineering.Pharmaceutics. 2025 Jan 13;17(1):98. doi: 10.3390/pharmaceutics17010098. Pharmaceutics. 2025. PMID: 39861746 Free PMC article. Review.
-
Silicon Enhances Functional Mitochondrial Transfer to Improve Neurovascularization in Diabetic Bone Regeneration.Adv Sci (Weinh). 2025 May;12(19):e2415459. doi: 10.1002/advs.202415459. Epub 2025 Mar 24. Adv Sci (Weinh). 2025. PMID: 40125794 Free PMC article.
-
Multifunctional Hydrogel with Synergistic Reactive Oxygen Species Scavenging and Macrophage Polarization-Induced Osteo-immunomodulation for Enhanced Bone Regeneration.ACS Appl Mater Interfaces. 2025 Jul 9;17(27):38985-39001. doi: 10.1021/acsami.5c08737. Epub 2025 Jun 24. ACS Appl Mater Interfaces. 2025. PMID: 40552867 Free PMC article.
-
A novel strategy for bone defect repair: Stromal cell-derived factor 1α sustained-release acellular fish scale scaffolds combined with injection of bone marrow mesenchymal stem cells promote bone regeneration.Mater Today Bio. 2025 Apr 10;32:101759. doi: 10.1016/j.mtbio.2025.101759. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40270891 Free PMC article.
-
Chiral gold nanoparticles manipulate osteoimmune microenvironment via macrophage autophagy for bone regeneration.Mater Today Bio. 2025 Jul 25;34:102131. doi: 10.1016/j.mtbio.2025.102131. eCollection 2025 Oct. Mater Today Bio. 2025. PMID: 40786657 Free PMC article.
References
-
- Xiao L, Ma Y, Crawford R, Mendhi J, Zhang Y, Lu H. et al. The interplay between hemostasis and immune response in biomaterial development for osteogenesis. Mater Today (Kidlington) 2022;54:202–24.
-
- Lao A, Wu J, Li D, Shen A, Li Y, Zhuang Y. et al. Functionalized Metal-Organic Framework-Modified Hydrogel That Breaks the Vicious Cycle of Inflammation and ROS for Repairing of Diabetic Bone Defects. Small. 2023;19:e2206919. - PubMed
-
- Xu N, Gao Y, Li Z, Chen Y, Liu M, Jia J. et al. Immunoregulatory hydrogel decorated with Tannic acid/Ferric ion accelerates diabetic wound healing via regulating Macrophage polarization. Chem Eng J. 2023;466:143173.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

