Structures of SenB and SenA enzymes from Variovorax paradoxus provide insights into carbon-selenium bond formation in selenoneine biosynthesis
- PMID: 38994077
- PMCID: PMC11237966
- DOI: 10.1016/j.heliyon.2024.e32888
Structures of SenB and SenA enzymes from Variovorax paradoxus provide insights into carbon-selenium bond formation in selenoneine biosynthesis
Abstract
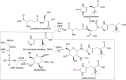
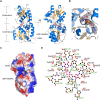
Selenoneine, an ergothioneine analog, is important for antioxidation and detoxification. SenB and SenA are two crucial enzymes that form carbon-selenium bonds in the selenoneine biosynthetic pathway. To investigate their underlying catalytic mechanisms, we obtained complex structures of SenB with its substrate UDP-N-acetylglucosamine (UDP-GlcNAc) and SenA with N-α-trimethyl histidine (TMH). SenB adopts a type-B glycosyltransferase fold. Structural and functional analysis of the interaction network at the active center provide key information on substrate recognition and suggest a metal-ion-independent, inverting mechanism is utilized for SenB-mediated selenoglycoside formation. Moreover, the complex structure of SenA with TMH and enzymatic activity assays highlight vital residues that control substrate binding and specificity. Based on the conserved structure and substrate-binding pocket of the type I sulfoxide synthase EgtB in the ergothioneine biosynthetic pathway, a similar reaction mechanism was proposed for the formation of C-Se bonds by SenA. The structures provide knowledge on selenoneine synthesis and lay groundwork for further applications of this pathway.
Keywords: Carbon–selenium bond; Ergothioneine; Glycosyltransferase; Selenoneine; Selenoneine synthase; SenA; SenB.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
LinkOut - more resources
Full Text Sources

