Differential peptide-dependent regulation of growth hormone (GH): A comparative analysis in pituitary cultures of reptiles, birds, and mammals
- PMID: 38994081
- PMCID: PMC11238054
- DOI: 10.1016/j.heliyon.2024.e33060
Differential peptide-dependent regulation of growth hormone (GH): A comparative analysis in pituitary cultures of reptiles, birds, and mammals
Abstract
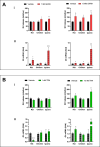
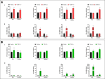
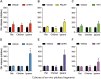
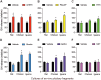
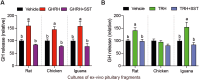
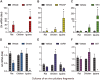
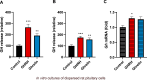
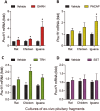
Growth hormone (GH) is a pituitary protein that exerts pleiotropic roles in vertebrates. The mechanisms regulating GH synthesis and secretion are finely controlled by hypothalamic neuropeptides and other factors. These processes have been considerably studied in mammals but are still poorly understood in other groups. To better understand the pituitary GH regulation during vertebrate phylogeny, we compared the effects of incubating several peptides on cultures of ex-vivo pituitary fragments obtained from representative specimens of reptiles (iguana), birds (chicken) and mammals (rat). The peptides used were: growth hormone-releasing hormone (GHRH), thyrotropin-releasing hormone (TRH), pituitary adenylate cyclase-activating polypeptide (PACAP), ghrelin, gonadotropin-releasing hormone (GnRH), and somatostatin (SST). In rat pituitary cultures, GH secretion was stimulated by GHRH and TRH, while gh mRNA expression was increased by GHRH and PACAP. In the case of chicken pituitaries, GH release was promoted by GHRH, ghrelin, PACAP, and GnRH, although the latter two had a dual effect since at a shorter incubation time they decreased GH secretion; in turn, gh mRNA expression was significantly stimulated by TRH, PACAP, and GnRH. The most intense effects were observed in iguana pituitary cultures, where GH secretion was significantly augmented by GHRH, PACAP, TRH, ghrelin, and GnRH; while gh mRNA expression was stimulated by GHRH, TRH, and PACAP, but inhibited by ghrelin and SST. Also, in the three species, SST was able to block the GHRH-stimulated GH release. Furthermore, it was found that the expression of Pou1f1 mRNA was increased with greater potency by GHRH and PACAP in the iguana, than in chicken or rat pituitary cultures. Additionally, in-silico analysis of the gh gene promoter structures in the three species showed that the reptilian promoter has more Pit-1 consensus binding sites than their avian and mammalian counterparts. Taken together, results demonstrate that pituitary peptide-mediated GH regulatory mechanisms are differentially controlled along vertebrate evolution.
Keywords: GH-Regulatory peptides; Growth hormone; Iguana; Pit-1; Pituitary.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Characterization and distribution of GHRH, PACAP, TRH, SST and IGF1 mRNAs in the green iguana.Gen Comp Endocrinol. 2018 Jan 1;255:90-101. doi: 10.1016/j.ygcen.2017.09.027. Epub 2017 Sep 30. Gen Comp Endocrinol. 2018. PMID: 28974369
-
Thyrotropin-Releasing Hormone (TRH) and Somatostatin (SST), but not Growth Hormone-Releasing Hormone (GHRH) nor Ghrelin (GHRL), Regulate Expression and Release of Immune Growth Hormone (GH) from Chicken Bursal B-Lymphocyte Cultures.Int J Mol Sci. 2020 Feb 20;21(4):1436. doi: 10.3390/ijms21041436. Int J Mol Sci. 2020. PMID: 32093298 Free PMC article.
-
Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal polypeptide (VIP) on hormone secretion from sheep pituitary cells in vitro.J Neuroendocrinol. 1997 Apr;9(4):279-86. doi: 10.1046/j.1365-2826.1997.00580.x. J Neuroendocrinol. 1997. PMID: 9147291
-
Molecular evolution of the growth hormone-releasing hormone/pituitary adenylate cyclase-activating polypeptide gene family. Functional implication in the regulation of growth hormone secretion.J Mol Endocrinol. 2000 Oct;25(2):157-68. doi: 10.1677/jme.0.0250157. J Mol Endocrinol. 2000. PMID: 11013344 Review.
-
Understanding the multifactorial control of growth hormone release by somatotropes: lessons from comparative endocrinology.Ann N Y Acad Sci. 2009 Apr;1163:137-53. doi: 10.1111/j.1749-6632.2008.03660.x. Ann N Y Acad Sci. 2009. PMID: 19456335 Review.
Cited by
-
GH inhibits ALV-J replication and restricts cell cycle by activating PI3K/Akt signaling pathway.Poult Sci. 2025 Jan;104(1):104514. doi: 10.1016/j.psj.2024.104514. Epub 2024 Nov 13. Poult Sci. 2025. PMID: 39586129 Free PMC article.
References
-
- Dehkhoda F., Lee C., Medina J., Brooks A. The growth hormone receptor: mechanism of receptor activation, cell signaling, and physiological aspects. Review Front Endocrinol (Lausanne) 2018;13:9–35. https://doi:10.3389/fendo.2018.00035 - DOI - PMC - PubMed
-
- Harvey S. In: Growth Hormone. Harvey S., Scanes C.G., Daughaday W.H., editors. CRC Press, Inc.; 1994. Growth hormone synthesis; pp. 55–78. ISBN 0-8493-8697-7.
LinkOut - more resources
Full Text Sources

