Search for unknown neural link between the masticatory and cognitive brain systems to clarify the involvement of its impairment in the pathogenesis of Alzheimer's disease
- PMID: 38994328
- PMCID: PMC11236757
- DOI: 10.3389/fncel.2024.1425645
Search for unknown neural link between the masticatory and cognitive brain systems to clarify the involvement of its impairment in the pathogenesis of Alzheimer's disease
Abstract
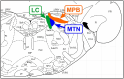
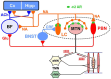
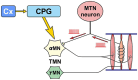
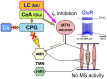
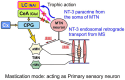
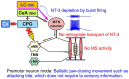
Brain degenerations in sporadic Alzheimer's disease (AD) are observed earliest in the locus coeruleus (LC), a population of noradrenergic neurons, in which hyperphosphorylated tau protein expression and β-amyloid (Aβ) accumulation begin. Along with this, similar changes occur in the basal forebrain cholinergic neurons, such as the nucleus basalis of Meynert. Neuronal degeneration of the two neuronal nuclei leads to a decrease in neurotrophic factors such as brain-derived neurotrophic factor (BDNF) in the hippocampus and cerebral cortex, which results in the accumulation of Aβ and hyperphosphorylated tau protein and ultimately causes neuronal cell death in those cortices. On the other hand, a large number of epidemiological studies have shown that tooth loss or masticatory dysfunction is a risk factor for dementia including AD, and numerous studies using experimental animals have also shown that masticatory dysfunction causes brain degeneration in the basal forebrain, hippocampus, and cerebral cortex similar to those observed in human AD, and that learning and memory functions are impaired accordingly. However, it remains unclear how masticatory dysfunction can induce such brain degeneration similar to AD, and the neural mechanism linking the trigeminal nervous system responsible for mastication and the cognitive and memory brain system remains unknown. In this review paper, we provide clues to the search for such "missing link" by discussing the embryological, anatomical, and physiological relationship between LC and its laterally adjoining mesencephalic trigeminal nucleus which plays a central role in the masticatory functions.
Keywords: 3,4-dihydroxyphenylglycolaldehyde (DOPEGAL); Alzheimer’s disease; locus coeruleus; mesencephalic trigeminal nucleus; neurotrophic factor-3 (NT-3).
Copyright © 2024 Kang, Toyoda and Saito.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Neurodegeneration of Trigeminal Mesencephalic Neurons by the Tooth Loss Triggers the Progression of Alzheimer's Disease in 3×Tg-AD Model Mice.J Alzheimers Dis. 2020;76(4):1443-1459. doi: 10.3233/JAD-200257. J Alzheimers Dis. 2020. PMID: 32651317 Free PMC article.
-
Locus Coeruleus Dysfunction and Trigeminal Mesencephalic Nucleus Degeneration: A Cue for Periodontal Infection Mediated Damage in Alzheimer's Disease?Int J Environ Res Public Health. 2023 Jan 5;20(2):1007. doi: 10.3390/ijerph20021007. Int J Environ Res Public Health. 2023. PMID: 36673763 Free PMC article. Review.
-
Heightened Hippocampal β-Adrenergic Receptor Function Drives Synaptic Potentiation and Supports Learning and Memory in the TgF344-AD Rat Model during Prodromal Alzheimer's Disease.J Neurosci. 2021 Jun 30;41(26):5747-5761. doi: 10.1523/JNEUROSCI.0119-21.2021. Epub 2021 May 5. J Neurosci. 2021. PMID: 33952633 Free PMC article.
-
The cholinergic system in aging and neuronal degeneration.Behav Brain Res. 2011 Aug 10;221(2):555-63. doi: 10.1016/j.bbr.2010.11.058. Epub 2010 Dec 9. Behav Brain Res. 2011. PMID: 21145918 Review.
-
An experimental model of Braak's pretangle proposal for the origin of Alzheimer's disease: the role of locus coeruleus in early symptom development.Alzheimers Res Ther. 2019 Jul 3;11(1):59. doi: 10.1186/s13195-019-0511-2. Alzheimers Res Ther. 2019. PMID: 31266535 Free PMC article.
Cited by
-
Topic Issue: "Translational Advances in Neurodegenerative Dementias".Neurol Int. 2025 Feb 19;17(2):31. doi: 10.3390/neurolint17020031. Neurol Int. 2025. PMID: 39997662 Free PMC article.
References
-
- Aigner T. G., Mitchell S. J., Aggleton J. P., Delong M. R., Struble R. G., Price D. L., et al. . (1987). Effects of scopolamine and physostigmine on recognition memory in monkeys with ibotenic-acid lesions of the nucleus basalis of Meynert. Psychopharmacology 92, 292–300. doi: 10.1007/BF00210833, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

