Professional phagocytes are recruited for the clearance of obsolete nonprofessional phagocytes in the Drosophila ovary
- PMID: 38994369
- PMCID: PMC11236694
- DOI: 10.3389/fimmu.2024.1389674
Professional phagocytes are recruited for the clearance of obsolete nonprofessional phagocytes in the Drosophila ovary
Abstract
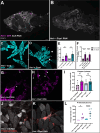
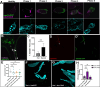
Cell death is an important process in the body, as it occurs throughout every tissue during development, disease, and tissue regeneration. Phagocytes are responsible for clearing away dying cells and are typically characterized as either professional or nonprofessional phagocytes. Professional phagocytes, such as macrophages, are found in nearly every part of the body while nonprofessional phagocytes, such as epithelial cells, are found in every tissue type. However, there are organs that are considered "immune-privileged" as they have little to no immune surveillance and rely on nonprofessional phagocytes to engulf dying cells. These organs are surrounded by barriers to protect the tissue from viruses, bacteria, and perhaps even immune cells. The Drosophila ovary is considered immune-privileged, however the presence of hemocytes, the macrophages of Drosophila, around the ovary suggests they may have a potential function. Here we analyze hemocyte localization and potential functions in response to starvation-induced cell death in the ovary. Hemocytes were found to accumulate in the oviduct in the vicinity of mature eggs and follicle cell debris. Genetic ablation of hemocytes revealed that the presence of hemocytes affects oogenesis and that they phagocytose ovarian cell debris and in their absence fecundity decreases. Unpaired3, an IL-6 like cytokine, was found to be required for the recruitment of hemocytes to the oviduct to clear away obsolete follicle cells. These findings demonstrate a role for hemocytes in the ovary, providing a more thorough understanding of phagocyte communication and cell clearance in a previously thought immune-privileged organ.
Keywords: Unpaired3; cell death; follicle; hemocyte; immune-privileged; nonprofessional phagocyte; ovary; phagocytosis.
Copyright © 2024 Chasse, Bandyadka, Wertheimer, Serizier and McCall.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
GATA factor Serpent promotes phagocytosis in non-professional phagocytes during Drosophila oogenesis.Development. 2025 May 1;152(9):dev204464. doi: 10.1242/dev.204464. Epub 2025 Apr 29. Development. 2025. PMID: 40136017
-
An RNAi screen of the kinome in epithelial follicle cells of the Drosophila melanogaster ovary reveals genes required for proper germline death and clearance.G3 (Bethesda). 2021 Feb 9;11(2):jkaa066. doi: 10.1093/g3journal/jkaa066. G3 (Bethesda). 2021. PMID: 33693600 Free PMC article.
-
Phagocyte Responses to Cell Death in Flies.Cold Spring Harb Perspect Biol. 2020 Apr 1;12(4):a036350. doi: 10.1101/cshperspect.a036350. Cold Spring Harb Perspect Biol. 2020. PMID: 31501193 Free PMC article. Review.
-
Protection of Insects against Viral Infection by Apoptosis-Dependent Phagocytosis.J Immunol. 2015 Dec 15;195(12):5696-706. doi: 10.4049/jimmunol.1500613. Epub 2015 Nov 6. J Immunol. 2015. PMID: 26546607
-
Murder on the Ovarian Express: A Tale of Non-Autonomous Cell Death in the Drosophila Ovary.Cells. 2021 Jun 10;10(6):1454. doi: 10.3390/cells10061454. Cells. 2021. PMID: 34200604 Free PMC article. Review.
Cited by
-
Ecdysone regulates phagocytic cell fate of epithelial cells in developing Drosophila eggs.J Cell Biol. 2025 Aug 4;224(8):e202411073. doi: 10.1083/jcb.202411073. Epub 2025 May 28. J Cell Biol. 2025. PMID: 40434296
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

