Controlling rates and reversibilities of elimination reactions of hydroxybenzylammoniums by tuning dearomatization energies
- PMID: 38994402
- PMCID: PMC11234877
- DOI: 10.1039/d4sc02985b
Controlling rates and reversibilities of elimination reactions of hydroxybenzylammoniums by tuning dearomatization energies
Abstract
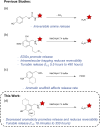
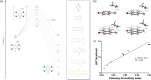
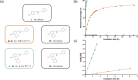
Hydroxybenzylammonium compounds can undergo a reversible 1,4- or 1,6-elimination to afford quinone methide intermediates after release of the amine. These molecules are useful for the reversible conjugation of payloads to amines. We hypothesized that aromaticity could be used to alter the rate of reversibility as a distinct thermodynamic driving force. We describe the use of density functional theory (DFT) calculations to determine the effect of aromaticity on the rate of release of the amine from hydroxybenzylammonium compounds. Namely, the aromatic scaffold affects the dearomatization reaction to reduce the kinetic barrier and prevent the reversibility of the amine elimination. We consequently synthesized a small library of polycyclic hydroxybenzylammoniums, which resulted in a range of release half-lives from 18 minutes to 350 hours. The novel mechanistic insight provided herein significantly expands the range of release rates amenable to hydroxybenzylammonium-containing compounds. This work provides another way to affect the rate of payload release in hydroxybenzylammoniums.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
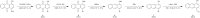


References
Grants and funding
LinkOut - more resources
Full Text Sources

