Hsp90α forms condensate engaging client proteins with RG motif repeats
- PMID: 38994413
- PMCID: PMC11234873
- DOI: 10.1039/d4sc00267a
Hsp90α forms condensate engaging client proteins with RG motif repeats
Abstract
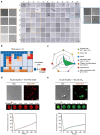
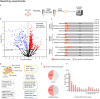
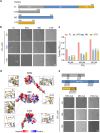
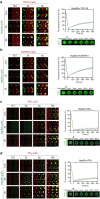
Hsp90α, a pivotal canonical chaperone, is renowned for its broad interaction with numerous protein clients to maintain protein homeostasis, chromatin remodeling, and cell growth. Recent studies indicate its role in modifying various components of membraneless organelles (MLOs) such as stress granules and processing bodies, suggesting its participation in the regulation of protein condensates. In this study, we found that Hsp90α possesses an inherent ability to form dynamic condensates in vitro. Utilizing LC-MS/MS, we further pinpointed proteins in cell lysates that preferentially integrate into Hsp90α condensates. Significantly, we observed a prevalence of RG motif repeats in client proteins of Hsp90α condensates, many of which are linked to various MLOs. Moreover, each of the three domains of Hsp90α was found to undergo phase separation, with numerous solvent-exposed negatively charged residues on these domains being crucial for driving Hsp90α condensation through multivalent weak electrostatic interactions. Additionally, various clients like TDP-43 and hnRNPA1, along with poly-GR and PR dipeptide repeats, exhibit varied impacts on the dynamic behavior of Hsp90α condensates. Our study spotlights various client proteins associated with Hsp90α condensates, illustrating its intricate adaptive nature in interacting with diverse clients and its functional adaptability across multiple MLOs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Xu Q. Ma Y. Sun Y. Li D. Zhang X. Liu C. Protein amyloid aggregate: structure and function. Aggregate. 2023;4:e333. doi: 10.1002/agt2.333. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

