Divergent functionalization of alkenes enabled by photoredox activation of CDFA and α-halo carboxylic acids
- PMID: 38994427
- PMCID: PMC11234866
- DOI: 10.1039/d4sc01084a
Divergent functionalization of alkenes enabled by photoredox activation of CDFA and α-halo carboxylic acids
Abstract
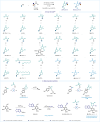
Herein we present our studies on the solvent-controlled difunctionalization of alkenes utilizing chlorodifluoroacetic acid (CDFA) and α-halo carboxylic acids for the synthesis of γ-lactones, γ-lactams and α,α-difluoroesters. Mechanistic insights revealed that photocatalytic reductive mesolytic cleavage of the C-X bond delivers elusive α-carboxyl alkyl radicals. In the presence of an olefin molecule, this species acts as a unique bifunctional intermediate allowing for stipulated formation of C-O, C-N and C-H bonds on Giese-type adducts via single electron transfer (SET) or hydrogen atom transfer (HAT) events. These protocols exhibit great efficiency across a broad spectrum of readily available α-halo carboxylic acids and are amenable to scalability in both batch and flow. To demonstrate the versatility of this concept, the synthesis of (±)-boivinianin A, its fluorinated analog and eupomatilone-6 natural products was successfully accomplished.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Photoredox Activation of Anhydrides for the Solvent-Controlled Switchable Synthesis of gem-Difluoro Compounds.Angew Chem Int Ed Engl. 2022 Oct 17;61(42):e202209143. doi: 10.1002/anie.202209143. Epub 2022 Sep 15. Angew Chem Int Ed Engl. 2022. PMID: 35997088 Free PMC article.
-
α-Amino Acids and Peptides as Bifunctional Reagents: Carbocarboxylation of Activated Alkenes via Recycling CO2.J Am Chem Soc. 2021 Feb 24;143(7):2812-2821. doi: 10.1021/jacs.0c11896. Epub 2021 Feb 9. J Am Chem Soc. 2021. PMID: 33561344
-
Photocatalytic Multisite Functionalization of Unactivated Terminal Alkenes by Merging Polar Cycloaddition and Radical Ring-Opening Process.Angew Chem Int Ed Engl. 2024 Oct 7;63(41):e202407928. doi: 10.1002/anie.202407928. Epub 2024 Sep 5. Angew Chem Int Ed Engl. 2024. PMID: 39022842
-
Difunctionalization of Alkenes and Alkynes via Intermolecular Radical and Nucleophilic Additions.Molecules. 2020 Dec 28;26(1):105. doi: 10.3390/molecules26010105. Molecules. 2020. PMID: 33379397 Free PMC article. Review.
-
Shining Light on C-S Bonds: Recent Advances in C-C Bond Formation Reactions via C-S Bond Cleavage under Photoredox Catalysis.Chem Asian J. 2020 Nov 16;15(22):3637-3659. doi: 10.1002/asia.202000905. Epub 2020 Oct 14. Chem Asian J. 2020. PMID: 32990368 Review.
Cited by
-
Visible-Light-Mediated Vicinal Dihalogenation of Unsaturated C-C Bonds Using Dual-Functional Group Transfer Reagents.J Am Chem Soc. 2024 Nov 20;146(46):31547-31559. doi: 10.1021/jacs.4c09039. Epub 2024 Nov 5. J Am Chem Soc. 2024. PMID: 39498866 Free PMC article.
-
Fluorinated Radicals in Divergent Synthesis via Photoredox Catalysis.Acc Chem Res. 2025 Jul 1;58(13):2046-2060. doi: 10.1021/acs.accounts.5c00239. Epub 2025 Jun 11. Acc Chem Res. 2025. PMID: 40499907 Free PMC article.
-
Overcoming Challenges in Small-Ring Transfer: Direct Decarboxylative Hydroalkylation of Alkenes via Iron-Thiol Catalysis.Angew Chem Int Ed Engl. 2025 Aug 18;64(34):e202508377. doi: 10.1002/anie.202508377. Epub 2025 Jul 7. Angew Chem Int Ed Engl. 2025. PMID: 40555684 Free PMC article.
-
Copper-catalysed oxy-alkylation of styrenes enabled by halogen-atom transfer.Chem Sci. 2025 Aug 26. doi: 10.1039/d5sc03774c. Online ahead of print. Chem Sci. 2025. PMID: 40896313 Free PMC article.
-
Phenothiazine Sulfoxides as Active Photocatalysts for the Synthesis of γ-Lactones.J Am Chem Soc. 2025 Apr 16;147(15):12908-12916. doi: 10.1021/jacs.5c01988. Epub 2025 Apr 2. J Am Chem Soc. 2025. PMID: 40174889 Free PMC article.

