A Pd-catalyzed route to carborane-fused boron heterocycles
- PMID: 38994428
- PMCID: PMC11234826
- DOI: 10.1039/d4sc02214a
A Pd-catalyzed route to carborane-fused boron heterocycles
Abstract
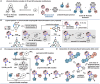
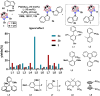
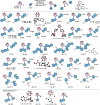
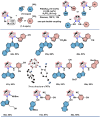
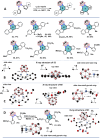
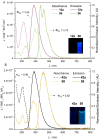
Due to the expanding applications of icosahedral carboranes in medicinal and materials chemistry research, their functionalizations have become one of the central themes in boron-rich cluster chemistry. Although several strategies for incorporating nitrogen-containing nucleophiles on a single boron vertex of the icosahedral carboranes (C2B10H12) have been developed, methods for preparing clusters with vicinal B-N moieties are still lacking. The steric bulk of icosahedral carboranes and disparate electronic and steric nature of the N-containing groups have rendered the vicinal diamination challenging. In this article, we show how a developed Pd-catalyzed process is used to incorporate an array of NH-heterocycles, anilines, and heteroanilines with various electronic and steric profiles onto the vicinal boron vertices of a meta-carborane cluster via sequential or one-pot fashion. Importantly, oxidative cyclizations of the cross-coupling products with indoles and pyrroles appended to boron vertices generate a previously unknown class of all-boron-vertex bound carborane-fused six- and seven-membered ring heterocycles. Photophysical studies of the meta-carborane-fused heterocycles show that these structures can exhibit luminescence with high quantum yields and are amenable to further manipulations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Synthesis, structure, and reactivity of 13- and 14-vertex carboranes.Acc Chem Res. 2014 May 20;47(5):1623-33. doi: 10.1021/ar500091h. Epub 2014 Apr 29. Acc Chem Res. 2014. PMID: 24773592
-
A Strategy for Selective Catalytic B-H Functionalization of o-Carboranes.Acc Chem Res. 2021 Nov 2;54(21):4065-4079. doi: 10.1021/acs.accounts.1c00460. Epub 2021 Oct 24. Acc Chem Res. 2021. PMID: 34693715
-
Improved synthesis of icosahedral carboranes containing exopolyhedral B-C and C-C bonds.Tetrahedron. 2019 Jan 11;75(2):187-191. doi: 10.1016/j.tet.2018.11.040. Epub 2018 Nov 28. Tetrahedron. 2019. PMID: 31303685 Free PMC article.
-
Carboranes as unique pharmacophores in antitumor medicinal chemistry.Mol Ther Oncolytics. 2022 Jan 10;24:400-416. doi: 10.1016/j.omto.2022.01.005. eCollection 2022 Mar 17. Mol Ther Oncolytics. 2022. PMID: 35141397 Free PMC article. Review.
-
Carborane-based BODIPY dyes: synthesis, structural analysis, photophysics and applications.Front Chem. 2024 Nov 5;12:1485301. doi: 10.3389/fchem.2024.1485301. eCollection 2024. Front Chem. 2024. PMID: 39564434 Free PMC article. Review.
Cited by
-
Post-coordination of Ru(ii) controlled regioselective B(4)-H acylmethylation of o-carboranes with sulfoxonium ylides.Chem Sci. 2025 Apr 24;16(21):9406-9412. doi: 10.1039/d5sc01576f. eCollection 2025 May 28. Chem Sci. 2025. PMID: 40308949 Free PMC article.
-
Beyond the Hawthorne Reaction: Li+ Induced Thermal Dehydrocoupling of closo-10-vertex Carborane Anions.Inorg Chem. 2025 Jan 13;64(1):757-760. doi: 10.1021/acs.inorgchem.4c04644. Epub 2024 Dec 24. Inorg Chem. 2025. PMID: 39719019 Free PMC article.
References
-
-
For reviews on generation of aryl C–N bonds see:
- Terrier F., Modern Nucleophilic Aromatic Substitution, Wiley-VCH, Weinheim, Germany, 2013
- Sambiagio C. Marsden S. P. Blacker A. J. McGowan P. C. Chem. Soc. Rev. 2014;43:3525–3550. doi: 10.1039/C3CS60289C. - DOI - PubMed
- Ruiz-Castillo P. Buchwald S. L. Chem. Rev. 2016;116:12564–12649. doi: 10.1021/acs.chemrev.6b00512. - DOI - PMC - PubMed
- Dorel R. Grugel C. P. Haydl A. M. Angew. Chem., Int. Ed. 2019;58:17118–17129. doi: 10.1002/anie.201904795. - DOI - PubMed
-
. For recent examples of 1,2-aryl diamine synthesis, see:
- Gonell S. Poyatos M. Peris E. Angew. Chem., Int. Ed. 2013;52:7009–7013. doi: 10.1002/anie.201302686. - DOI - PubMed
- Li L. Qiu D. Shi J. Li Y. Org. Lett. 2016;18:3726–3729. doi: 10.1021/acs.orglett.6b01747. - DOI - PubMed
- Xiong M. Gao Z. Liang X. Cai P. Zhu H. Pan Y. Chem. Commun. 2018;54:9679–9682. doi: 10.1039/C8CC05320K. - DOI - PubMed
- Sánchez-Bento R. Roure B. Llaveria J. Ruffoni A. Leonori D. Chem. 2023;9:3685–3695. doi: 10.1016/j.chempr.2023.10.008. - DOI
-
-
-
For recent examples of vicinal diaminated arenes in medicinal research, see:
- O'Neill D. J. Adedoyin A. Bray J. A. Deecher D. C. Fensome A. Goldberg J. A. Harrison J. Leventhal L. Mann C. Mark L. Nogle L. Sullivan N. R. Spangler T. B. Terefenko E. A. Trybulski E. J. Uveges A. J. Vu A. Whiteside G. T. Zhang P. J. Med. Chem. 2011;54:6824–6831. doi: 10.1021/jm200733r. - DOI - PubMed
- Karaman M. W. Herrgard S. Treiber D. K. Gallant P. Atteridge C. E. Campbell B. T. Chan K. W. Ciceri P. Davis M. I. Edeen P. T. Faraoni R. Floyd M. Hunt J. P. Lockhart D. J. Milanov Z. V. Morrison M. J. Pallares G. Patel H. K. Pritchard S. Wodicka L. M. Zarrinkar P. P. Nat. Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. - DOI - PubMed
-
. For reviews and recent applications of ortho-phenylene diamines in functional polymers, see:
- Li X.-G. Huang M.-R. Duan W. Yang Y.-L. Chem. Rev. 2002;102:2925–3030. doi: 10.1021/cr010423z. - DOI - PubMed
- Guan Q. L. Sun Y. Huo R. Xin Y. Bai F. Y. Xing Y. H. Sun L. X. Inorg. Chem. 2021;60:2829–2838. doi: 10.1021/acs.inorgchem.0c03753. - DOI - PubMed
- Zhang H. Lam S. H. Guo Y. Yang J. Lu Y. Shao L. Yang B. Xiao L. Wang J. ACS Appl. Mater. Interfaces. 2021;13:51855–51866. doi: 10.1021/acsami.1c03806. - DOI - PubMed
- Kim S.-W. Lee W. K. Lee J.-S. ACS Omega. 2023;8:46267–46275. doi: 10.1021/acsomega.3c07669. - DOI - PMC - PubMed
-
-
- Lipscomb W. N., Boron Hydrides, Benjamin, New York, 1963
- Schleyer P. V. R. Najafian K. Inorg. Chem. 1998;37:3454–3470. doi: 10.1021/ic980110v. - DOI - PubMed
- Chen Z. King R. B. Chem. Rev. 2005;105:3613–3642. doi: 10.1021/cr0300892. - DOI - PubMed
- King R. B. Chem. Rev. 2001;101:1119–1152. doi: 10.1021/cr000442t. - DOI - PubMed
LinkOut - more resources
Full Text Sources

