Spearmint targets microtubules by (-)-carvone
- PMID: 38994449
- PMCID: PMC11237191
- DOI: 10.1093/hr/uhae151
Spearmint targets microtubules by (-)-carvone
Abstract
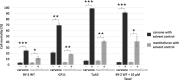
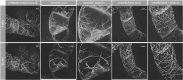
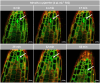
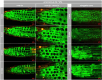
Allelopathy can provide sustainable alternatives to herbicides because it is based on specific signals rather than generic toxicity. We show that the allelopathic activity of Spearmint and Watermint is linked with their main compounds, (-)-carvone and (+)-menthofuran, both deriving from (-)-limonene. Germination of Poppy and Cress, and root growth of Arabidopsis thaliana are inhibited by very low concentrations of (-)-carvone, acting even through the gas phase. (+)-Menthofuran is active as well, but at lower efficacy. Using fluorescently tagged marker lines in tobacco BY-2 cells and Arabidopsis roots, we demonstrate a rapid degradation of microtubules and a remodeling of actin filaments in response to (-)-carvone and, to a milder extent, to (+)-menthofuran. This cytoskeletal response is followed by cell death. By means of a Root Chip system, we can follow the tissue dependent response of the cytoskeleton and show a cell-type dependent gradient of sensitivity between meristem and distal elongation zone, accompanied by programmed cell death.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nanjing Agricultural University.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Molisch H. Der Einfluß einer Pflanze auf die andere. Allelopathie. Jena: Gustav Fischer; 1937.
-
- Einhellig FA. Allelopathy: current status and future goals. ACS Symp Ser. 1995;582:1–24.
-
- Rizvi SJH, Haque H, Singh VK et al. A discipline called allelopathy. In: Rizvi SJH, Rizvi V, eds. Allelopathy: Basic and applied aspects. Springer: Dordrecht, 1992,1–10.
-
- Von Bertalanffy L. The theory of open Systems in Physics and Biology. Science. 1950;111:23–9. - PubMed
-
- Karban R, Maron J, Felton GW et al. Herbivore damage to sagebrush induces resistance in wild tobacco: evidence for eavesdropping between plants. Oikos. 2003;100:325–32.
LinkOut - more resources
Full Text Sources

