Therapeutic Impact of Tocilizumab in the Setting of Severe COVID-19; an Updated and Comprehensive Review on Current Evidence
- PMID: 38994467
- PMCID: PMC11239185
- DOI: 10.22037/aaem.v12i1.2217
Therapeutic Impact of Tocilizumab in the Setting of Severe COVID-19; an Updated and Comprehensive Review on Current Evidence
Abstract
Introduction: The COVID-19 pandemic caused by SARS-CoV-2 has been the major health concern in 2019 globally. Considering the severity and phase of the disease, various pharmacotherapy schedules were proposed. Here, we set out to provide close-up insights on the clinical utility of Tocilizumab (TCZ), a biologic monoclonal antibody in this regard.
Methods: In this comprehensive review, various databases, including Scopus, PubMed Central, Medline, Embase, Google Scholar, and preprint publishers (med/bioRxiv) were searched until January 30, 2024, according to the keywords and search criteria.
Results: Besides the pros and cons, compelling evidence purported the safety and efficacy of TCZ and indicated that it exhibits great potential to reduce short-term and all-cause (28-30-day) mortality. TCZ significantly drops the adverse events if administered in the right time course (in the inflammatory phase) during critical/severe COVID-19 pneumonia. Despite contradictory results, the benefits of TCZ appear significant, especially in combination with add-on therapies, such as corticosteroids. Although the safety of TCZ is acceptable, solid data is lacking as to its benefits during pregnancy. There are limited data on TCZ combination therapies, such as hemoperfusion, intravenous immunoglobulin (IVIG), simple O2 therapy, vasopressor support, convalescent plasma therapy, and even in vaccinated patients and COVID-19 reinfection, especially in elderly persons. In addition, the impact of TCZ therapy on the long-lasting COVID-19 is unclear.
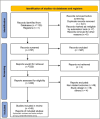
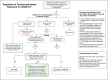
Conclusion: Personalized medicine based on individual characteristics and pertinent clinical conditions must be considered in the clinicians' decision-making policy. Finally, to mitigate the risk-to-benefit ratio of TCZ, a treatment algorithm, based on available literature and updated national institute of health (NIH) and Infectious Diseases Society of America (IDSA) guidelines, is also proposed.
Keywords: Algorithms; COVID-19; COVID-19 drug treatment; SARS-CoV-2; Tocilizumab; Treatment outcome.
Conflict of interest statement
The authors of this manuscript declared that they have no conflict of interest.
Figures


References
-
- Rezabakhsh A, Ala A, Khodaei SH. Novel coronavirus (COVID-19): a new emerging pandemic threat. J Res Clin Med. 2020;8:5.
-
- Tahsini Tekantapeh S, Ghojazadeh M, Ghamari AA, Mohammadi A, Soleimanpour H. Therapeutic and anti-inflammatory effects of baricitinib on mortality, ICU transfer, clinical improvement, and CRS-related laboratory parameters of hospitalized patients with moderate to severe COVID-19 pneumonia: a systematic review and meta-analysis. Expert Rev Respir Med. 2022;16(10):1109–32. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
