Conformational dynamics of CasX (Cas12e) in mediating DNA cleavage revealed by single-molecule FRET
- PMID: 38994558
- PMCID: PMC11347132
- DOI: 10.1093/nar/gkae604
Conformational dynamics of CasX (Cas12e) in mediating DNA cleavage revealed by single-molecule FRET
Abstract
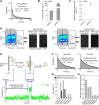
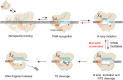
CasX (also known as Cas12e), a Class 2 CRISPR-Cas system, shows promise in genome editing due to its smaller size compared to the widely used Cas9 and Cas12a. Although the structures of CasX-sgRNA-DNA ternary complexes have been resolved and uncover a distinctive NTSB domain, the dynamic behaviors of CasX are not well characterized. In this study, we employed single-molecule and biochemical assays to investigate the conformational dynamics of two CasX homologs, DpbCasX and PlmCasX, from DNA binding to target cleavage and fragment release. Our results indicate that CasX cleaves the non-target strand and the target strand sequentially with relative irreversible dynamics. The two CasX homologs exhibited different cleavage patterns and specificities. The dynamic characterization of CasX also reveals a PAM-proximal seed region, providing guidance for CasX-based effector design. Further studies elucidate the mechanistic basis for why modification of sgRNA and the NTSB domain can affect its activity. Interestingly, CasX has less effective target search efficiency than Cas9 and Cas12a, potentially accounting for its lower genome editing efficiency. This observation opens a new avenue for future protein engineering.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
Chimeric CRISPR-CasX enzymes and guide RNAs for improved genome editing activity.Mol Cell. 2022 Mar 17;82(6):1199-1209.e6. doi: 10.1016/j.molcel.2022.02.002. Epub 2022 Feb 25. Mol Cell. 2022. PMID: 35219382 Free PMC article.
-
Position of Deltaproteobacteria Cas12e nuclease cleavage sites depends on spacer length of guide RNA.RNA Biol. 2020 Oct;17(10):1472-1479. doi: 10.1080/15476286.2020.1777378. Epub 2020 Jun 21. RNA Biol. 2020. PMID: 32564655 Free PMC article.
-
Mechanistic Insights into the cis- and trans-Acting DNase Activities of Cas12a.Mol Cell. 2019 Feb 7;73(3):589-600.e4. doi: 10.1016/j.molcel.2018.11.021. Epub 2019 Jan 10. Mol Cell. 2019. PMID: 30639240 Free PMC article.
-
Editor's cut: DNA cleavage by CRISPR RNA-guided nucleases Cas9 and Cas12a.Biochem Soc Trans. 2020 Feb 28;48(1):207-219. doi: 10.1042/BST20190563. Biochem Soc Trans. 2020. PMID: 31872209 Free PMC article. Review.
-
Anti-CRISPRs: Protein Inhibitors of CRISPR-Cas Systems.Annu Rev Biochem. 2020 Jun 20;89:309-332. doi: 10.1146/annurev-biochem-011420-111224. Epub 2020 Mar 18. Annu Rev Biochem. 2020. PMID: 32186918 Free PMC article. Review.
Cited by
-
Single-molecule two- and three-colour FRET studies reveal a transition state in SNARE disassembly by NSF.Nat Commun. 2025 Jan 2;16(1):250. doi: 10.1038/s41467-024-55531-0. Nat Commun. 2025. PMID: 39747074 Free PMC article.
-
Cas12e orthologs evolve variable structural elements to facilitate dsDNA cleavage.Nat Commun. 2024 Dec 30;15(1):10727. doi: 10.1038/s41467-024-54491-9. Nat Commun. 2024. PMID: 39737904 Free PMC article.
-
PlmCas12e Utilizes Glu662 to Prevent Cleavage Site Occupation by Positively Charged Residues Before Target Strand Cleavage.Molecules. 2024 Oct 25;29(21):5036. doi: 10.3390/molecules29215036. Molecules. 2024. PMID: 39519677 Free PMC article.
-
Single-molecule perspectives of CRISPR/Cas systems: target search, recognition, and cleavage.BMB Rep. 2025 Jan;58(1):8-16. doi: 10.5483/BMBRep.2024-0182. BMB Rep. 2025. PMID: 39701024 Free PMC article. Review.
References
-
- Horvath P., Barrangou R.. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010; 327:167–170. - PubMed
-
- Marraffini L.A. CRISPR-Cas immunity in prokaryotes. Nature. 2015; 526:55–61. - PubMed
-
- van Beljouw S.P.B., Sanders J., Rodríguez-Molina A., Brouns S.J.J.. RNA-targeting CRISPR-Cas systems. Nat. Rev. Microbiol. 2023; 21:21–34. - PubMed
-
- Wright A.V., Nunez J.K., Doudna J.A.. Biology and applications of CRISPR systems: harnessing Nature's toolbox for genome engineering. Cell. 2016; 164:29–44. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

