Steroid sulfatase and sulfotransferases in the estrogen and androgen action of gynecological cancers: current status and perspectives
- PMID: 38994718
- PMCID: PMC11625860
- DOI: 10.1042/EBC20230096
Steroid sulfatase and sulfotransferases in the estrogen and androgen action of gynecological cancers: current status and perspectives
Abstract
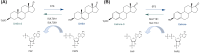
Sulfatase (STS) and sulfotransferases (SULT) have important role in the biosynthesis and action of steroid hormones. STS catalyzes the hydrolysis of estrone-sulfate (E1-S) and dehydroepiandrosterone-sulfate (DHEA-S), while sulfotransferases catalyze the reverse reaction and require 3-phosphoadenosine-5-phosphosulfate as a sulfate donor. These enzymes control the concentration of active estrogens and androgens in peripheral tissues. Aberant expression of STS and SULT genes has been found in both, benign hormone-dependent diseases and hormone-dependent cancers. The aim of this review is to present the current knowledge on the role of STS and SULT in gynecological cancers, endometrial (EC) and ovarian cancer (OC). EC is the most common and OC the most lethal gynecological cancer. These cancers primarily affect postmenopausal women and therefore rely on the local production of steroid hormones from inactive precursors, either DHEA-S or E1-S. Following cellular uptake by organic anion transporting polypeptides (OATP) or organic anion transporters (OAT), STS and SULT regulate the formation of active estrogens and androgens, thus disturbed balance between STS and SULT can contribute to the onset and progression of cancer. The importance of these enzymes in peripheral estrogen biosynthesis has long been recognized, and this review provides new data on the important role of STS and SULT in the formation and action of androgens, their regulation and inhibition, and their potential as prognostic biomarkers.
Keywords: androgens; estrogens; intracrinology; sulfatase; sulfotransferase.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



Similar articles
-
New development in intracrinology of breast carcinoma.Breast Cancer. 2006;13(2):129-36. doi: 10.2325/jbcs.13.129. Breast Cancer. 2006. PMID: 16755106 Review.
-
Expression of 17beta-hydroxysteroid dehydrogenases and other estrogen-metabolizing enzymes in different cancer cell lines.Chem Biol Interact. 2009 Mar 16;178(1-3):228-33. doi: 10.1016/j.cbi.2008.10.038. Epub 2008 Nov 5. Chem Biol Interact. 2009. PMID: 19022235
-
The Important Roles of Steroid Sulfatase and Sulfotransferases in Gynecological Diseases.Front Pharmacol. 2016 Feb 18;7:30. doi: 10.3389/fphar.2016.00030. eCollection 2016. Front Pharmacol. 2016. PMID: 26924986 Free PMC article. Review.
-
Steroid sulfatase and estrogen sulfotransferase in human carcinomas.Mol Cell Endocrinol. 2011 Jul 4;340(2):148-53. doi: 10.1016/j.mce.2010.11.001. Epub 2010 Nov 10. Mol Cell Endocrinol. 2011. PMID: 21073915 Review.
-
Aromatase and steroid sulfatase from human placenta.Methods Enzymol. 2023;689:67-86. doi: 10.1016/bs.mie.2023.04.025. Epub 2023 May 25. Methods Enzymol. 2023. PMID: 37802583 Review.
Cited by
-
Precision Therapeutic and Preventive Molecular Strategies for Endometriosis-Associated Infertility.Int J Mol Sci. 2025 Aug 9;26(16):7706. doi: 10.3390/ijms26167706. Int J Mol Sci. 2025. PMID: 40869026 Free PMC article. Review.
-
Sulfation pathways in times of change.Essays Biochem. 2024 Dec 4;68(4):379-382. doi: 10.1042/EBC20230099. Essays Biochem. 2024. PMID: 39630031 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

