The components of an electrical synapse as revealed by expansion microscopy of a single synaptic contact
- PMID: 38994821
- PMCID: PMC11333041
- DOI: 10.7554/eLife.91931
The components of an electrical synapse as revealed by expansion microscopy of a single synaptic contact
Abstract
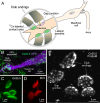
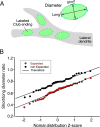
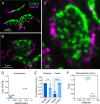
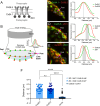
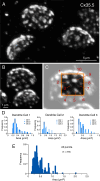
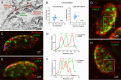
Most nervous systems combine both transmitter-mediated and direct cell-cell communication, known as 'chemical' and 'electrical' synapses, respectively. Chemical synapses can be identified by their multiple structural components. Electrical synapses are, on the other hand, generally defined by the presence of a 'gap junction' (a cluster of intercellular channels) between two neuronal processes. However, while gap junctions provide the communicating mechanism, it is unknown whether electrical transmission requires the contribution of additional cellular structures. We investigated this question at identifiable single synaptic contacts on the zebrafish Mauthner cells, at which gap junctions coexist with specializations for neurotransmitter release and where the contact unequivocally defines the anatomical limits of a synapse. Expansion microscopy of these single contacts revealed a detailed map of the incidence and spatial distribution of proteins pertaining to various synaptic structures. Multiple gap junctions of variable size were identified by the presence of their molecular components. Remarkably, most of the synaptic contact's surface was occupied by interleaving gap junctions and components of adherens junctions, suggesting a close functional association between these two structures. In contrast, glutamate receptors were confined to small peripheral portions of the contact, indicating that most of the synaptic area functions as an electrical synapse. Thus, our results revealed the overarching organization of an electrical synapse that operates with not one, but multiple gap junctions, in close association with structural and signaling molecules known to be components of adherens junctions. The relationship between these intercellular structures will aid in establishing the boundaries of electrical synapses found throughout animal connectomes and provide insight into the structural organization and functional diversity of electrical synapses.
Keywords: N-cadherin; adherens junction; connexin; gap junction; glutamate; neuroscience; zebrafish; ß-catenin.
Plain language summary
Neurons communicate with each other through specialized structures known as synapses. At chemical synapses, the cells do not physically interact as they rely instead on molecules called neurotransmitters to pass along signals. At electrical synapses, however, neurons are directly connected via gap junctions, which are clusters of intercellular channels that allow ions and other small compounds to move from one cell to another. Both electrical and chemical synapses play critical roles in neural circuits, and both exhibit some amount of plasticity – they weaken or strengthen depending on how often they are used, an important feature for the brain to adapt to the needs of the environment. Yet the structure and molecular organization of electrical synapses have remained poorly understood compared to their chemical counterparts. In response, Cárdenas-García, Ijaz and Pereda took advantage of a new approach known as expansion microscopy to examine the electrical synapse that connects neurons bringing sound information to a pair of unusually large neurons in the brain of most bony fish. With this method, a biological sample is prepared in such a way that its size increases, but the relative position of its components is preserved. This allows scientists to better observe structures that would otherwise be too difficult to capture using traditional microscopy techniques. Experiments in larval zebrafish revealed that contrary to previous assumptions, the electrical synapse was formed of not one but multiple gap junctions of various sizes closely associated with a range of structural and signaling molecules typically found in adherens junctions (a type of structure that physically links cells together). The team suggests that these molecular actors could work to ensure that the multiple gap junctions act in concert at the synapse. Overall, these findings offer a new perspective on how electrical synapses are organized and regulated, which refines our understanding of how the nervous system functions both in health and in disease.
© 2024, Cárdenas-García et al.
Conflict of interest statement
SC, SI, AP No competing interests declared
Figures


Update of
-
The components of an electrical synapse as revealed by expansion microscopy of a single synaptic contact.bioRxiv [Preprint]. 2023 Jul 28:2023.07.25.550347. doi: 10.1101/2023.07.25.550347. bioRxiv. 2023. Update in: Elife. 2024 Jul 12;13:e91931. doi: 10.7554/eLife.91931. PMID: 37546897 Free PMC article. Updated. Preprint.
References
-
- Bartelmez GW. Mauthner’s cell and the nucleus motorius tegmenti. Journal of Comparative Neurology. 1915;25:87–128. doi: 10.1002/cne.900250105. - DOI
-
- Bartelmez GW, Hoerr NL. The vestibular club endings in ameiurus: further evidence on the morphology of the synapse. Journal of Comparative Neurology. 1933;57:401–428. doi: 10.1002/cne.900570303. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

