Mid-Infrared Photoacoustic Stimulation of Neurons through Vibrational Excitation in Polydimethylsiloxane
- PMID: 38994890
- PMCID: PMC11425203
- DOI: 10.1002/advs.202405677
Mid-Infrared Photoacoustic Stimulation of Neurons through Vibrational Excitation in Polydimethylsiloxane
Abstract
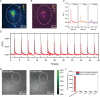
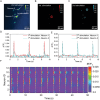
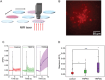
Photoacoustic (PA) emitters are emerging ultrasound sources offering high spatial resolution and ease of miniaturization. Thus far, PA emitters rely on electronic transitions of absorbers embedded in an expansion matrix such as polydimethylsiloxane (PDMS). Here, it is shown that mid-infrared vibrational excitation of C─H bonds in a transparent PDMS film can lead to efficient mid-infrared photoacoustic conversion (MIPA). MIPA shows 37.5 times more efficient than the commonly used PA emitters based on carbon nanotubes embedded in PDMS. Successful neural stimulation through MIPA both in a wide field with a size up to a 100 µm radius and in single-cell precision is achieved. Owing to the low heat conductivity of PDMS, less than a 0.5 °C temperature increase is found on the surface of a PDMS film during successful neural stimulation, suggesting a non-thermal mechanism. MIPA emitters allow repetitive wide-field neural stimulation, opening up opportunities for high-throughput screening of mechano-sensitive ion channels and regulators.
Keywords: mid‐infrared; neural stimulation; photoacoustic.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
C.Y. and J.X.C. serve as Scientific Advisor for Axorus. CY received a research grant from Axorus, which did not support this work. C.Y. and J.X.C. have a patent on Methods and Devices for Optoacoustic Stimulation (US Patent No. 11684404 B2) issued.
Figures



Similar articles
-
Photoacoustic: A Versatile Nongenetic Method for High-Precision Neuromodulation.Acc Chem Res. 2024 Jun 4;57(11):1595-1607. doi: 10.1021/acs.accounts.4c00119. Epub 2024 May 17. Acc Chem Res. 2024. PMID: 38759211 Free PMC article. Review.
-
High-Precision Photoacoustic Neural Modulation Uses a Non-Thermal Mechanism.Adv Sci (Weinh). 2024 Aug;11(32):e2403205. doi: 10.1002/advs.202403205. Epub 2024 Jun 26. Adv Sci (Weinh). 2024. PMID: 38923780 Free PMC article.
-
Polydimethylsiloxane thin film characterization using all-optical photoacoustic mechanism.Appl Opt. 2013 Sep 1;52(25):6239-44. doi: 10.1364/AO.52.006239. Appl Opt. 2013. PMID: 24085082
-
Nano-structural characteristics of carbon nanotube-polymer composite films for high-amplitude optoacoustic generation.Nanoscale. 2015 Sep 14;7(34):14460-8. doi: 10.1039/c5nr03769g. Nanoscale. 2015. PMID: 26255948
-
Photoacoustic imaging in the second near-infrared window: a review.J Biomed Opt. 2019 Apr;24(4):1-20. doi: 10.1117/1.JBO.24.4.040901. J Biomed Opt. 2019. PMID: 30968648 Free PMC article. Review.
Cited by
-
Spatial-offset pump-probe imaging of nonradiative dynamics at optical resolution.Sci Adv. 2025 Jul 4;11(27):eadw4939. doi: 10.1126/sciadv.adw4939. Epub 2025 Jul 2. Sci Adv. 2025. PMID: 40601728 Free PMC article.
References
-
- Ikeda T., Yoshizawa S., Koizumi N., Mitsuishi M., Matsumoto Y., in (Eds.: Escoffre J.‐M., Bouakaz A.), Springer International Publishing, Cham: 2016.
-
- a) Yu K., Niu X., Krook‐Magnuson E., He B., Nat. Commun. 2021, 12, 2519; - PMC - PubMed
- b) Rabut C., Yoo S., Hurt R. C., Jin Z., Li H., Guo H., Ling B., Shapiro M. G., Neuron 2020, 108, 93; - PMC - PubMed
- c) Jiang L., Lu G., Zeng Y., Sun Y., Kang H., Burford J., Gong C., Humayun M. S., Chen Y., Zhou Q., Nat. Commun. 2022, 13, 3853; - PMC - PubMed
- d) Fry W. J., Fry F. J., Barnard J. W., Krumins R. F., Brennan J. F., Science 1955, 122, 517. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
