Hyperactivation of mTOR/eIF4E Signaling Pathway Promotes the Production of Tryptophan-To-Phenylalanine Substitutants in EBV-Positive Gastric Cancer
- PMID: 38994917
- PMCID: PMC11425274
- DOI: 10.1002/advs.202402284
Hyperactivation of mTOR/eIF4E Signaling Pathway Promotes the Production of Tryptophan-To-Phenylalanine Substitutants in EBV-Positive Gastric Cancer
Abstract
Although messenger RNA translation is tightly regulated to preserve protein synthesis and cellular homeostasis, chronic exposure to interferon-γ (IFN-γ) in several cancers can lead to tryptophan (Trp) shortage via the indoleamine-2,3-dioxygenase (IDO)- kynurenine pathway and therefore promotes the production of aberrant peptides by ribosomal frameshifting and tryptophan-to-phenylalanine (W>F) codon reassignment events (substitutants) specifically at Trp codons. However, the effect of Trp depletion on the generation of aberrant peptides by ribosomal mistranslation in gastric cancer (GC) is still obscure. Here, it is shows that the abundant infiltrating lymphocytes in EBV-positive GC continuously secreted IFN-γ, upregulated IDO1 expression, leading to Trp shortage and the induction of W>F substitutants. Intriguingly, the production of W>F substitutants in EBV-positive GC is linked to antigen presentation and the activation of the mTOR/eIF4E signaling pathway. Inhibiting either the mTOR/eIF4E pathway or EIF4E expression counteracted the production and antigen presentation of W>F substitutants. Thus, the mTOR/eIF4E pathway exposed the vulnerability of gastric cancer by accelerating the production of aberrant peptides and boosting immune activation through W>F substitutant events. This work proposes that EBV-positive GC patients with mTOR/eIF4E hyperactivation may benefit from anti-tumor immunotherapy.
Keywords: EBV‐positive gastric cancer; aberrant mRNA translation; codon reassignment; substitutants; tryptophan.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
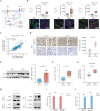

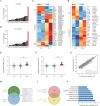




References
-
- Yuan S. Q., Nie R. C., Jin Y., Liang C. C., Li Y. F., Jian R., Sun X. W., Chen Y. B., Guan W. L., Wang Z. X., Qiu H. B., Wang W., Chen S., Zhang D. S., Ling Y. H., Xi S. Y., Cai M. Y., Huang C. Y., Yang Q. X., Liu Z. M., Guan Y. X., Chen Y. M., Li J. B., Tang X. W., Peng J. S., Zhou Z. W., Xu R. H., Wang F., Nat. Med. 2024, 30, 552. - PubMed
-
- Smyth E. C., Nilsson M., Grabsch H. I., van Grieken N. C., Lordick F., Lancet 2020, 396, 635. - PubMed
-
- Li K., Zhang A., Li X., Zhang H., Zhao L., Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188615. - PubMed
-
- Nature 2014, 513, 202. - PubMed
MeSH terms
Substances
Grants and funding
- 82303803/National Natural Science Foundation of China
- 82103586/National Natural Science Foundation of China
- 2023zgjh04/Chih Kuang Scholarship for Outstanding Young Physician-Scientists of Sun Yat-sen University Cancer Center
- 2022TQ0390/Postdoctoral Research Foundation of China
- 2022M723655/Postdoctoral Research Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
