Roxadustat Attenuates Adverse Remodeling Following Myocardial Infarction in Mice
- PMID: 38994928
- PMCID: PMC11240812
- DOI: 10.3390/cells13131074
Roxadustat Attenuates Adverse Remodeling Following Myocardial Infarction in Mice
Abstract
Activation of the CXCL12/CXCR4/ACKR3 axis is known to aid myocardial repair through ischemia-triggered hypoxia-inducible factor-1α (HIF-1α). To enhance the upregulation of HIF-1α, we administered roxadustat, a novel prolyl hydroxylase inhibitor (PHI) clinically approved by the European Medicines Agency 2021 for the treatment of renal anemia, with the purpose of improving LV function and attenuating ischemic cardiomyopathy.
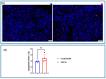
Methods: We evaluated roxadustat's impact on HIF-1 stimulation, cardiac remodeling, and function after MI. Therefore, we analyzed nuclear HIF-1 expression, the mRNA and protein expression of key HIF-1 target genes (RT-PCR, Western blot), inflammatory cell infiltration (immunohistochemistry), and apoptosis (TUNEL staining) 7 days after MI. Additionally, we performed echocardiography in male and female C57BL/6 mice 28 days post-MI.
Results: We found a substantial increase in nuclear HIF-1, associated with an upregulation of HIF-1α target genes like CXCL12/CXCR4/ACKR3 at the mRNA and protein levels. Roxadustat increased the proportion of myocardial reparative M2 CD206+ cells, suggesting beneficial alterations in immune cell migration and a trend towards reduced apoptosis. Echocardiography showed that roxadustat treatment significantly preserved ejection fraction and attenuated subsequent ventricular dilatation, thereby reducing adverse remodeling.
Conclusions: Our findings suggest that roxadustat is a promising clinically approved treatment option to preserve myocardial function by attenuating adverse remodeling.
Keywords: acute coronary syndrome; adverse remodeling; apoptosis; cardiomyopathy; chemokines; fibrosis; hypoxia inducible factor-1; inflammation; prolyl hydroxylase inhibitor.
Conflict of interest statement
Santhosh Kumar Ghadge was involved in research work at the Medical University Innsbruck and is now an employee of Valneva SE. Moritz Messner received funding from the “Tiroler Wissenschaftsförderung” with the grant number “TWF–F.18815”.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

