Bromodomain Protein Inhibition Protects β-Cells from Cytokine-Induced Death and Dysfunction via Antagonism of NF-κB Pathway
- PMID: 38994961
- PMCID: PMC11240345
- DOI: 10.3390/cells13131108
Bromodomain Protein Inhibition Protects β-Cells from Cytokine-Induced Death and Dysfunction via Antagonism of NF-κB Pathway
Abstract
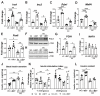
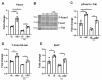
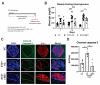
Cytokine-induced β-cell apoptosis is a major pathogenic mechanism in type 1 diabetes (T1D). Despite significant advances in understanding its underlying mechanisms, few drugs have been translated to protect β-cells in T1D. Epigenetic modulators such as bromodomain-containing BET (bromo- and extra-terminal) proteins are important regulators of immune responses. Pre-clinical studies have demonstrated a protective effect of BET inhibitors in an NOD (non-obese diabetes) mouse model of T1D. However, the effect of BET protein inhibition on β-cell function in response to cytokines is unknown. Here, we demonstrate that I-BET, a BET protein inhibitor, protected β-cells from cytokine-induced dysfunction and death. In vivo administration of I-BET to mice exposed to low-dose STZ (streptozotocin), a model of T1D, significantly reduced β-cell apoptosis, suggesting a cytoprotective function. Mechanistically, I-BET treatment inhibited cytokine-induced NF-kB signaling and enhanced FOXO1-mediated anti-oxidant response in β-cells. RNA-Seq analysis revealed that I-BET treatment also suppressed pathways involved in apoptosis while maintaining the expression of genes critical for β-cell function, such as Pdx1 and Ins1. Taken together, this study demonstrates that I-BET is effective in protecting β-cells from cytokine-induced dysfunction and apoptosis, and targeting BET proteins could have potential therapeutic value in preserving β-cell functional mass in T1D.
Keywords: Brd4; I-BET; NF-kB; STZ; apoptosis; bromodomain; cytokines; diabetes; inflammation; insulin; islet; β-cells.
Conflict of interest statement
There are no conflicts of interest.
Figures




References
-
- Fowler M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes. 2008;26:77–82. doi: 10.2337/diaclin.26.2.77. - DOI
-
- Goudy K., Song S., Wasserfall C., Zhang Y.C., Kapturczak M., Muir A., Powers M., Scott-Jorgensen M., Campbell-Thompson M., Crawford J.M., et al. Adeno-associated virus vector-mediated Il-10 gene delivery prevents type 1 diabetes in NOD mice. Proc. Natl. Acad. Sci. USA. 2001;98:13913–13918. doi: 10.1073/pnas.251532298. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

